Lesson 5, DNA: Translation
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
tRNA
Transfer RNA- carries amino acids to ribosomes; each molecule is specific for a different amino acid
- molecule that translates between nucleotide codons and amino acids
One end has an anticodon that contains the complement of that molecule's mRNA codon
codon
Three-nucleotide sequence coding for an amino acid.
universal code
Codons are shared by almost all organisms.
non-overlapping code
Codons are read sequentially without overlap.
degenerate/redundant code
Multiple codons can encode the same amino acid, but each codon can only code for one amino acid
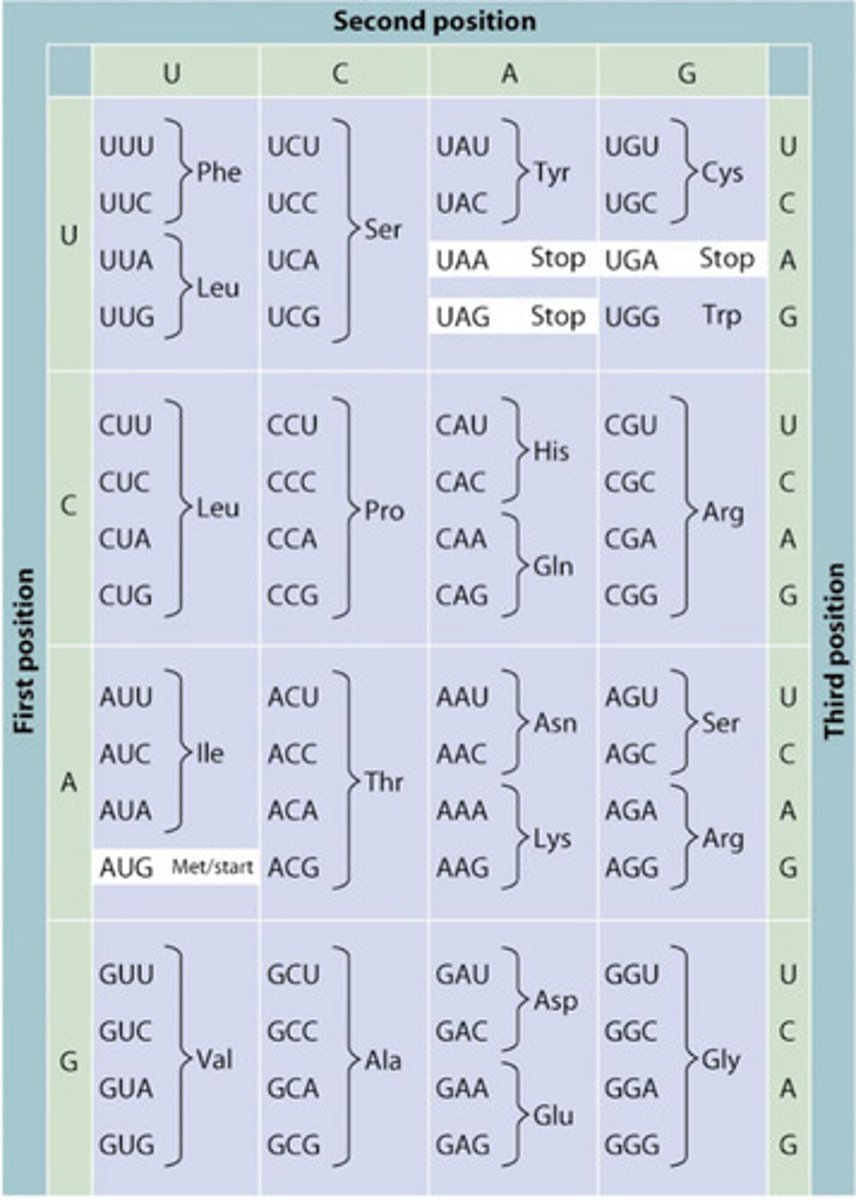
start codon
AUG
stop codon
UAA, UGA, UAG
Open reading frame
Determined by the start codon (AUG), dictates codon grouping; following AUG, every nucleotide will be grouped into successive, non-overlapping triplets.
anticodon
tRNA sequence complementary to mRNA codon
Each codon is recognized by a specific anticodon-
However, unusual pairings between the 1st nucleotide of the anticodon and 3rd nucleotide of the codon lead to 1 tRNA molecule being capable of recognizing several codons
wobble pairing
Refers to flexibility in the pairing between the base at the 5' end of a tRNA anticodon and the base at the 3' end of an mRNA codon. This flexibility allows a single tRNA to read more than one mRNA codon.
- This flexibility allows for tRNA molecules to bind to their complementary codon, even when the codon has been mutated (in the 3rd base)
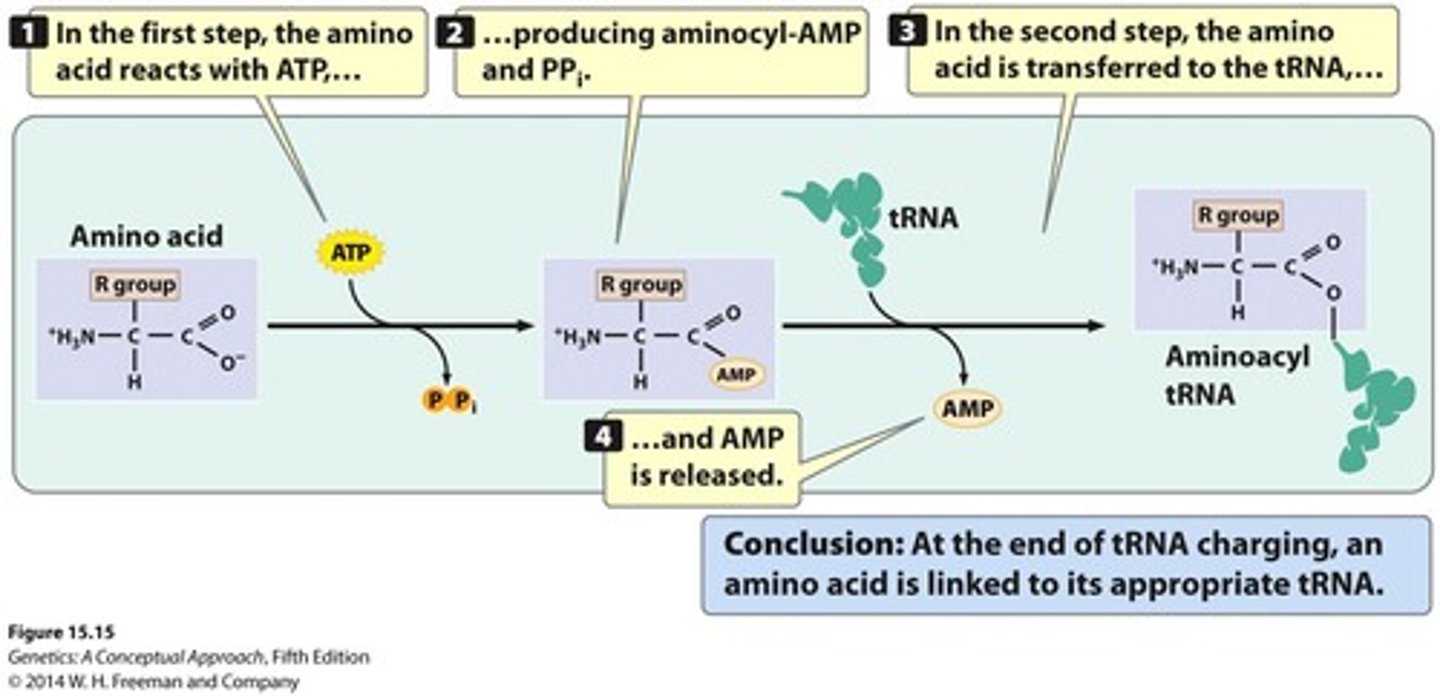
tRNA charging
TRNA loading with amino acid prior to use
- Requires ATP to generate an active ester bond ("charging")
-An enzyme specific to each tRNA (via sequences and structure of tRNA) attaches the corresponding amino acid to the tRNA molecule, so that the amino acid can be used at the ribosome.
If a tRNA is incorrectly charged with the wrong amino acid, it will be incorporated into the protein anyway
aminoacyl-tRNA synthetase
Enzyme that charges tRNA with the correct amino acid.
- each enzyme recognizes one amino acid and one tRNA
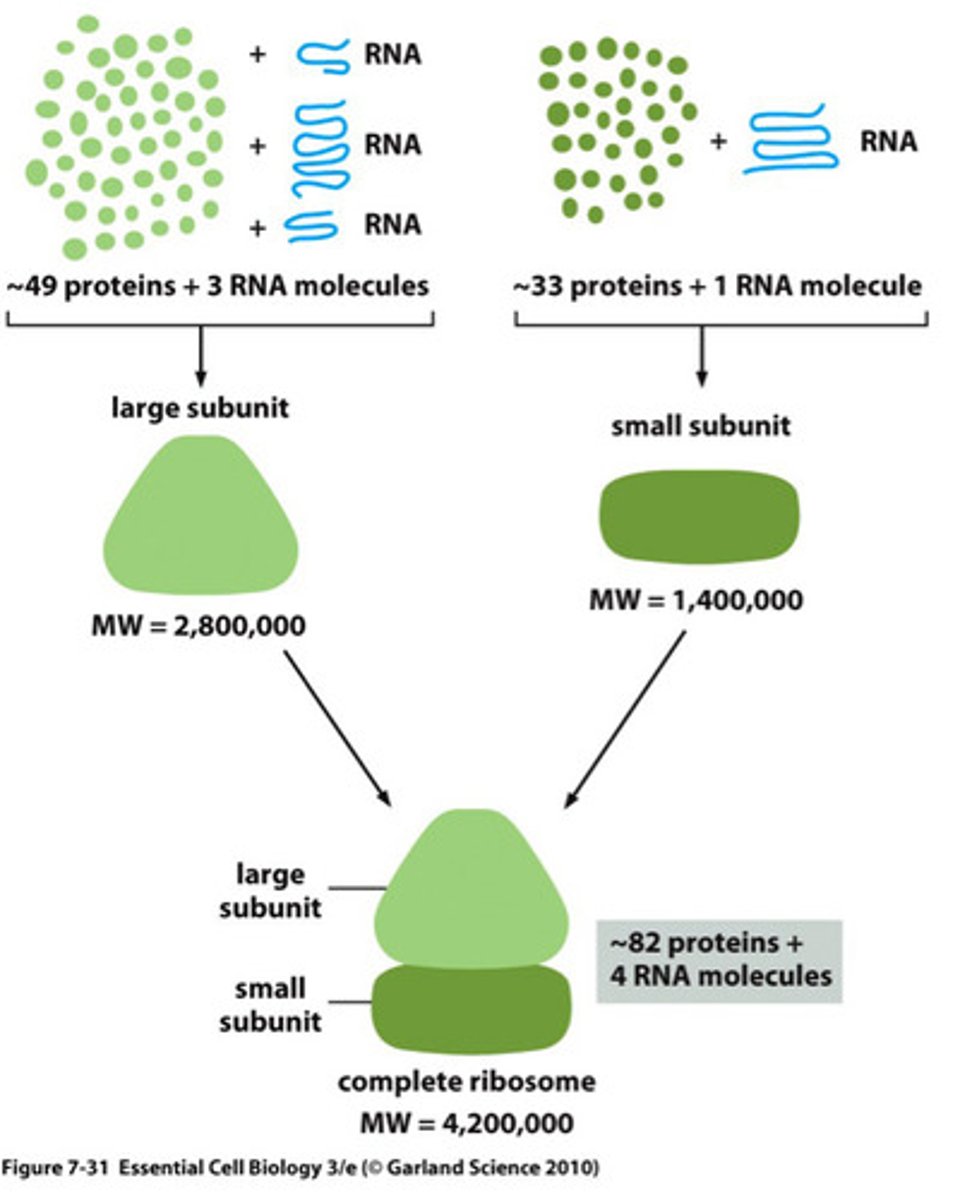
ribosome
Macromolecular complex that synthesizes proteins. This complex reads mRNA, aligns the tRNA anticodon with the complementary codon on the mRNA, and catalyzes peptide bond formation between each amino acid added by the tRNA
- Composed of RNA (structural and functional) and proteins and two subunits, large and small.
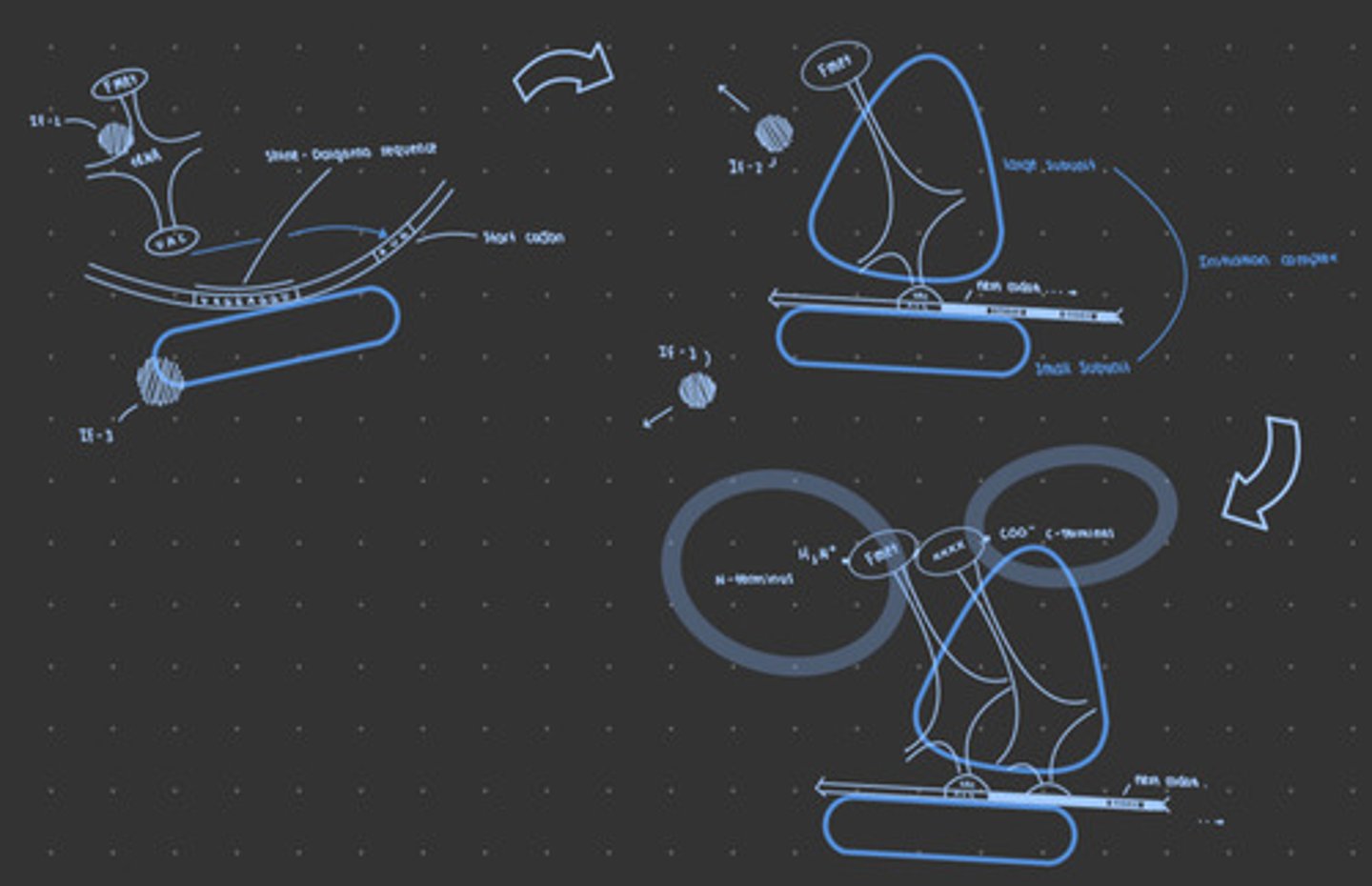
Initiation in bacteria
Initiation factor 3 (IF-3) binds to small ribosomal subunit and allows the small subunit to read along the mRNA. Once the small subunit encounters the Shine-Dalgarno sequence, in binds.
Initiation factor 2 (IF-2) brings fMET tRNA to the small subunit, where it associates with the start codon.
GTP hydrolysis allows for addition of large subunit, Initiation complex forms and peptide chain begins
N-terminus to C-terminus
direction of protein synthesis
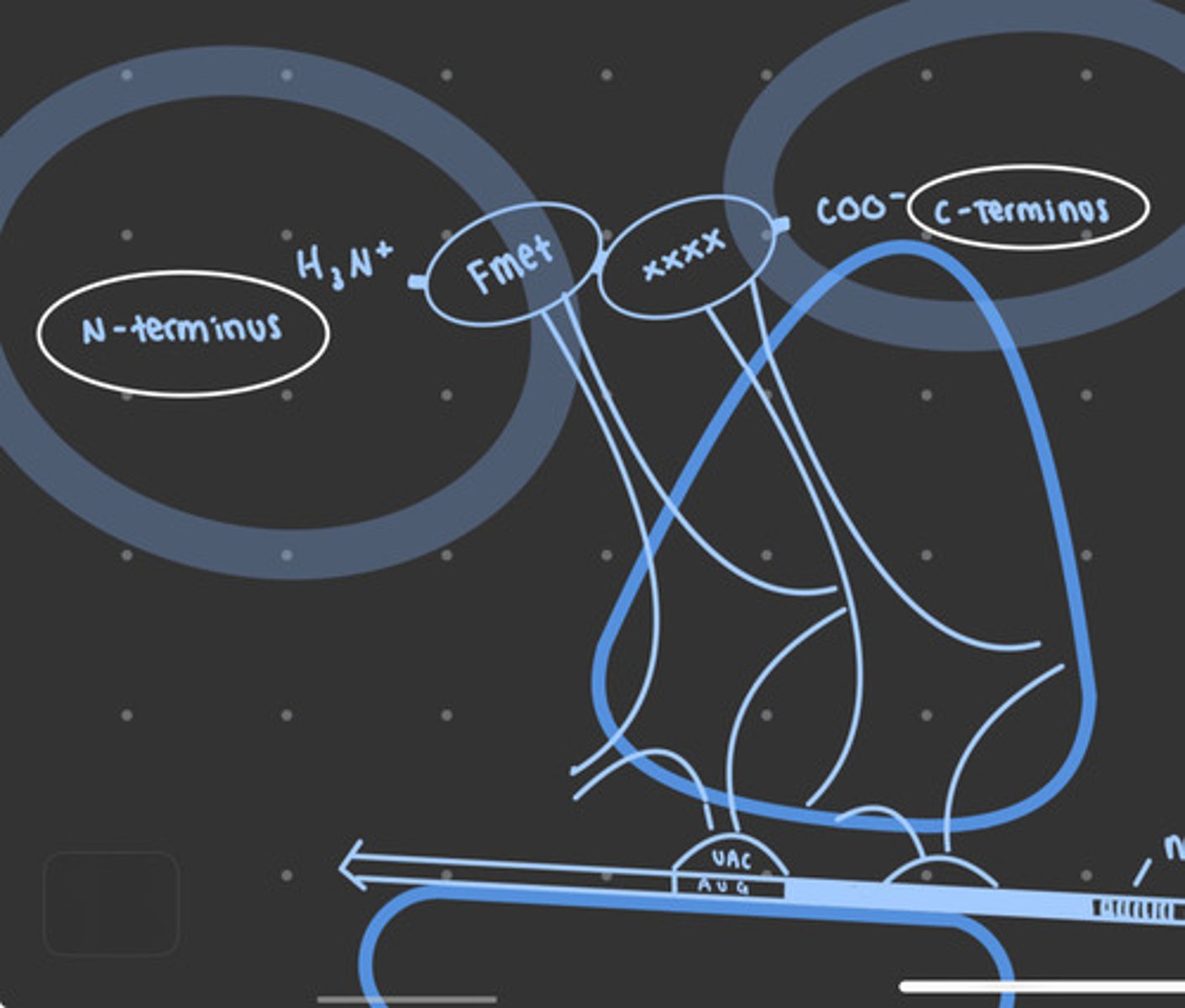
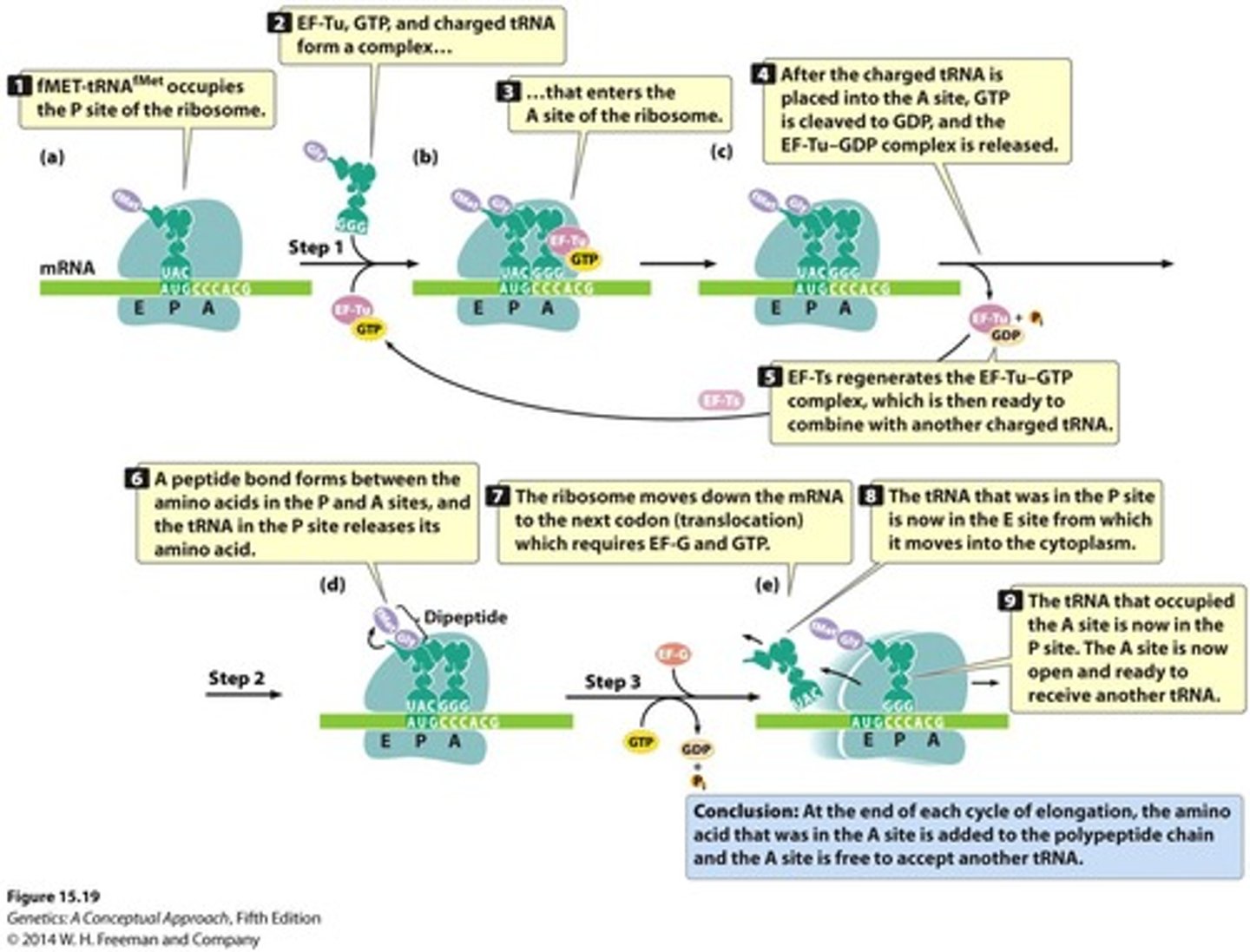
Elongation in bacteria
Elongation factors move ribosome along the mRNA to read a new codon, and the appropriate tRNA adds the corresponding amino acid, and the cycle repeats, adding one amino acid each time
initiation complex
Assembly of ribosomal subunits and mRNA for initiation of translation.
elongation factors
Proteins assisting in the elongation phase of translation.
Termination
Ribosome encounters a stop codon, which does not have a tRNA to pair with it.
Release factors bind to the stop codon and trigger the release of the new polypeptide
release factors
Proteins that bind to the stop codon of mRNA and promote termination of translation, release of the new polypeptide
Eukaryotic translation
- Start codon is regular met
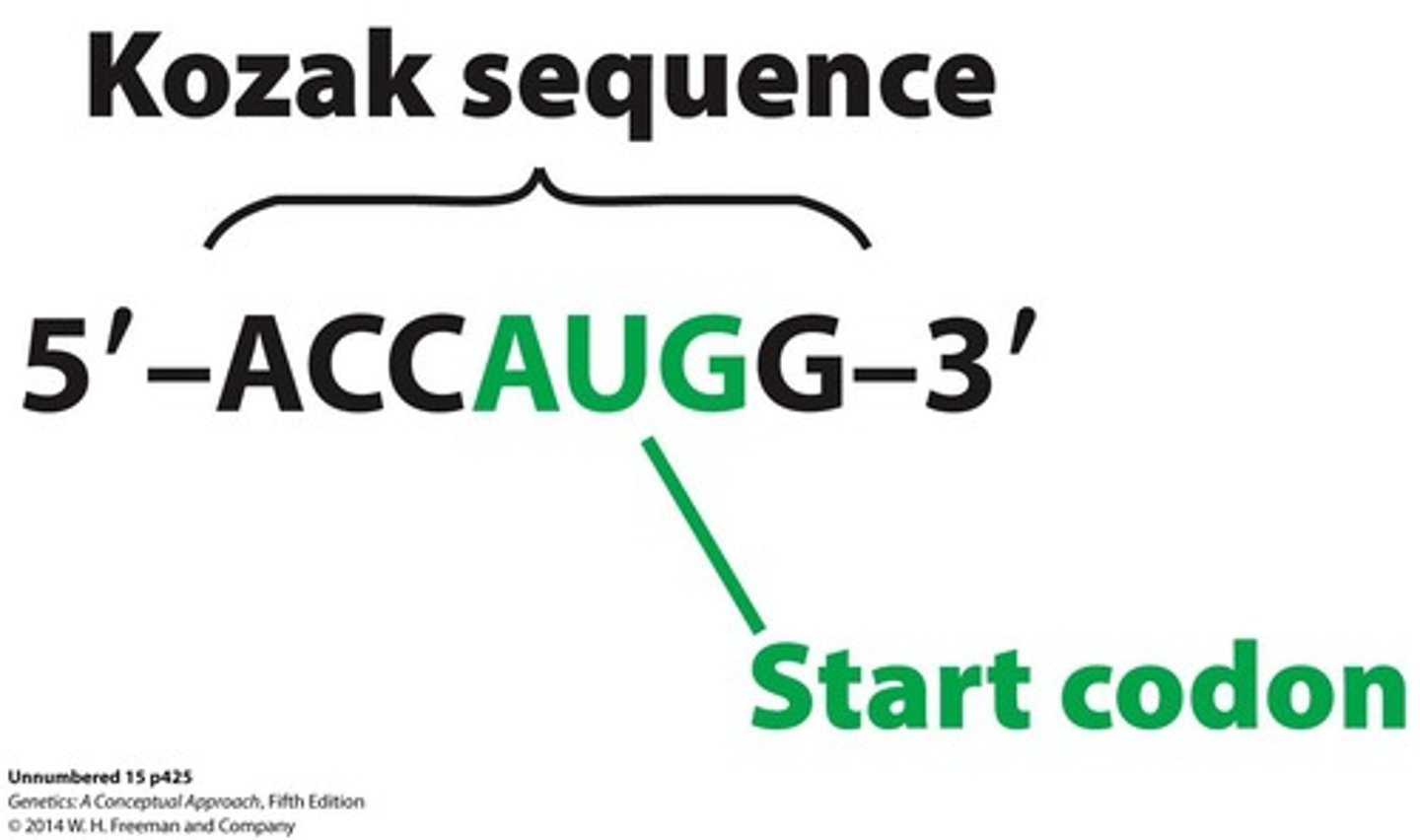
- start sequence is the Kozac sequence
- 5' cap, polyA tail, and UTR's play a role in translation
- Different initiation factors (IF) and elongation factors (EF) are used, but have similar roles
- transcription and translation are not coupled
Kozak sequence
Sequence that facilitates translation initiation in eukaryotes.
Shine-Dalgarno sequence
The sequence of initiation of translation in prokaryotes.
Protein functions
Proteins can be enzymes, transporters, structural, signaling, and storing. Function of the protein depends on its structure.
Side chain (R group) of protein determines interactions within the organism and affects its function.
genes must undergo transcription and translation to produce a functional protein
peptide bonds
Covalent bonds linking amino acids in proteins.
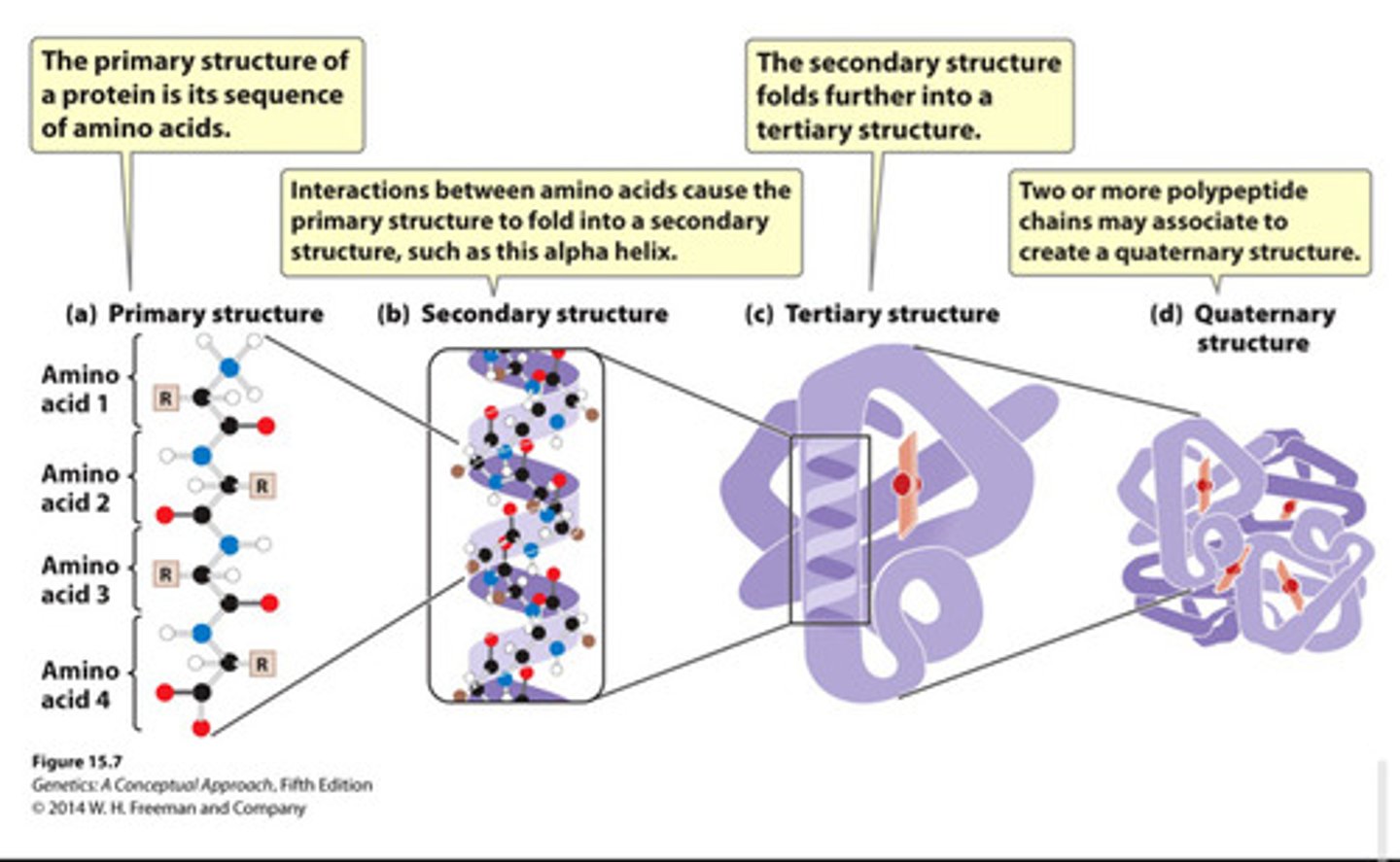
protein structure levels
Primary (linear sequence)
secondary (coiling or folding of a polypeptide due to H-bonding between amino acids)
tertiary (bending or coiling of secondary structure, stabilized by covalent, hydrogen, van der Waals)
and quaternary structures (association between two or more polypeptide chains within one protein)
-chemical interactions between amino acid side chains determine structure and function of the protein