Exam 1 Study Guide (Systems Physiology Dr. Nelson)
1/169
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
170 Terms
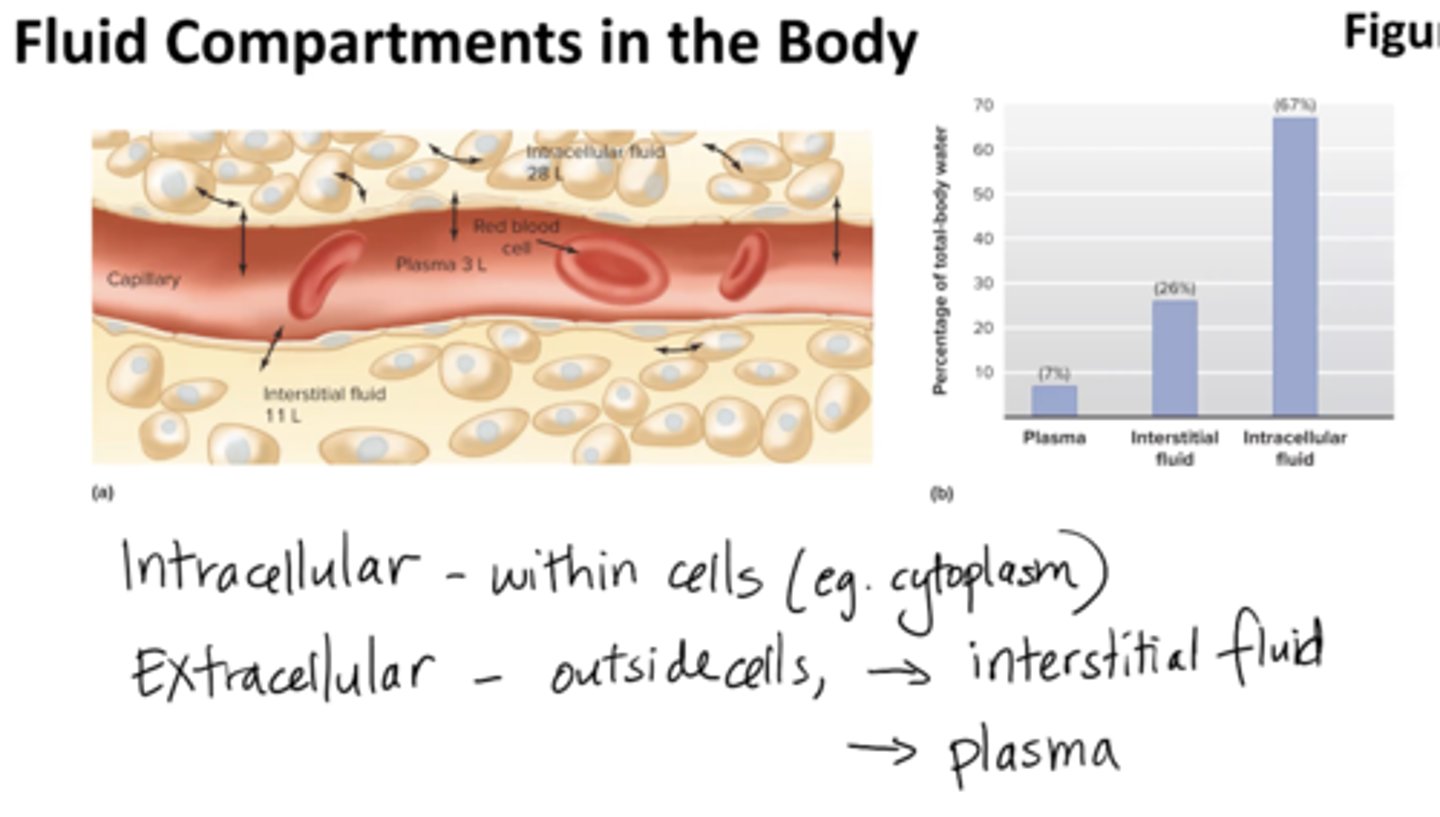
What are the fluid compartments of the body? Why do we care?
extracellular fluid (outside cells):
- plasma (liquid portion of blood)
- interstitial fluid
intracellular fluid:
contained within all the cells of the body, accounts for about
- 67% of all water in the body (cytoplasm)
ultimately determine physiology
Blood is a part of which fluid compartment?
plasma
T/F: Interstitial fluid and plasma are both considered extracellular fluid and thus have similar ionic components
true
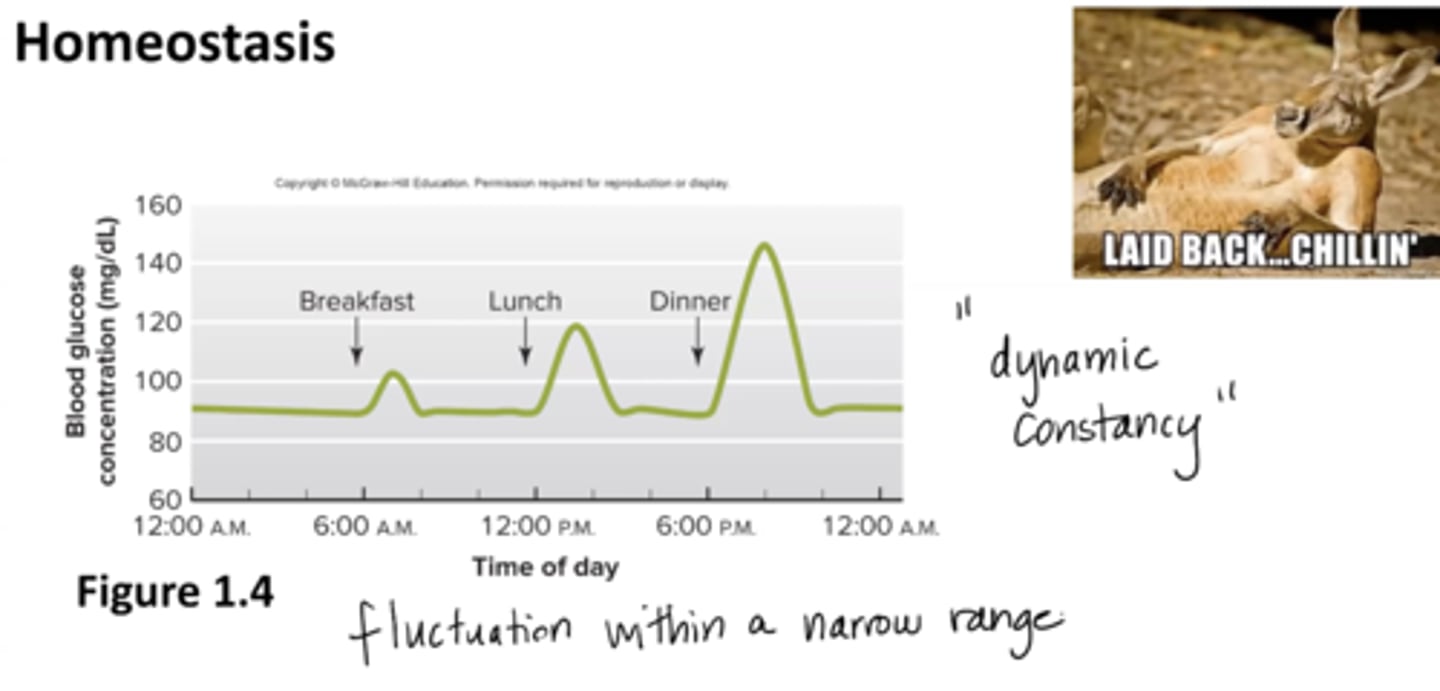
What is homeostasis?
dynamic constancy of the internal environment
fluctuation within a narrow range
maintained through negative feedback
negative feedback
increase or decrease in a variable results in a response that moves the variable in the opposite direction (returns to set point)
Ex: blood glucose, body temp, water + sodium
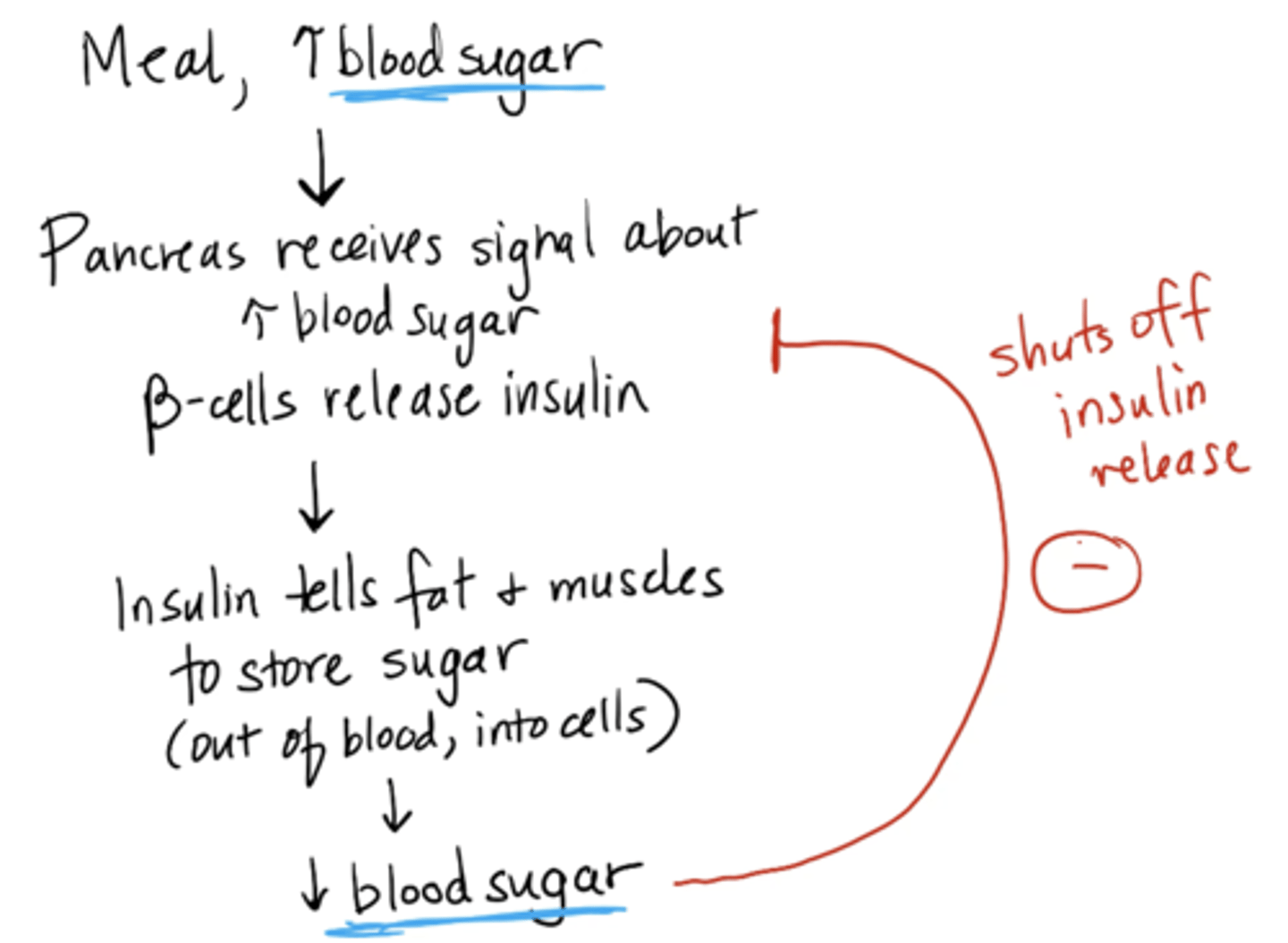
blood glucose mechanism
1. Meal → blood sugar increases
2. Pancreas receives signal about increase → beta cells release insulin
3. Insulin tells fat + muscles to store sugar (out of blood, into cells
4. blood glucose decreases (shuts off insulin release)
If the amount of sodium in the blood decreased, what would a negative feedback control mechanism be expect to do?
A) increase the amount of sodium in the blood
B) decrease the amount of sodium in the blood
C) leave the amount of sodium unchanged
D) change the set point for sodium
E) inhibit the ingestion of more sodium
A) increase the amount of sodium in the blood
positive feedback
accelerated process, explosive system that moves a variable further from a set point until stimulus is gone (less common)
Ex: labor and delivery, blood clotting, orgasm
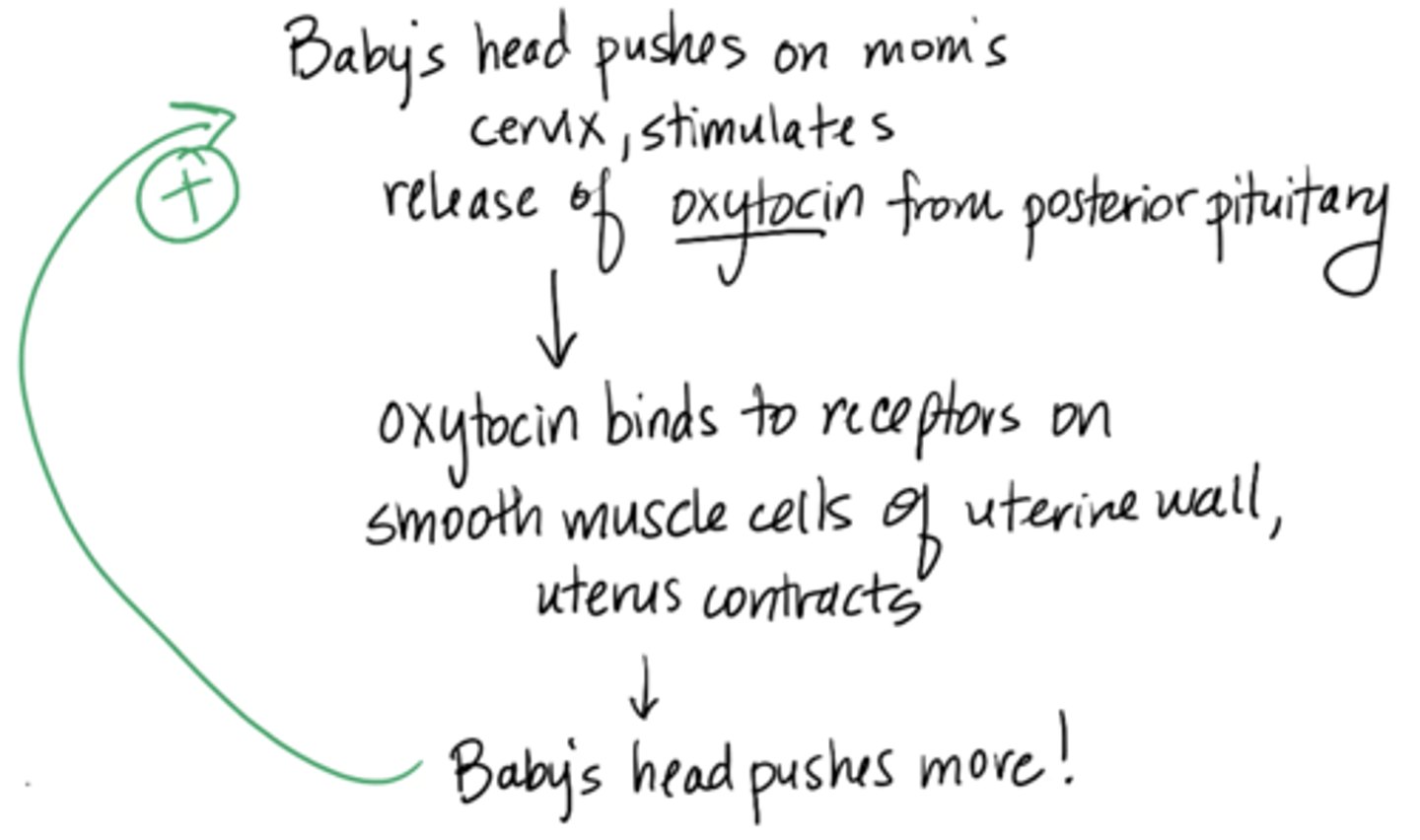
labor and delivery mechanism
1. Baby’s head pushes on mom’s cervix, stimulates release of oxytocin from posterior pituitary
2. oxytocin binds to receptors on smooth muscle cells of uterine wall, uterus contracts
3. baby’s head pushes more (continues until baby is out)
feedforward regulation
body anticipates changes and prepares for them
ex: butterflies and ozempic
Which of the following is an example of homeostasis?
A) you take a hot shower and your body temp increases
B) you go swimming in a cold lake and your body temp decreases
C) you overeat for a week and you gain weight
D) you run a marathon and you get thirsty
E) none of the above
D) you run a marathon and you get thirsty
this is the right answer bc you have an event (the marathon, which obviously will dehydrate you) and then you have the body's attempt to return to homeostasis by activating thirst mechanisms
if choice B said that you shivered, it would be correct too
T/F: Extracellular fluid, including intracellular and interstitial fluid, is high in potassium.
false

Nerve cells are most permeable to...
Potassium
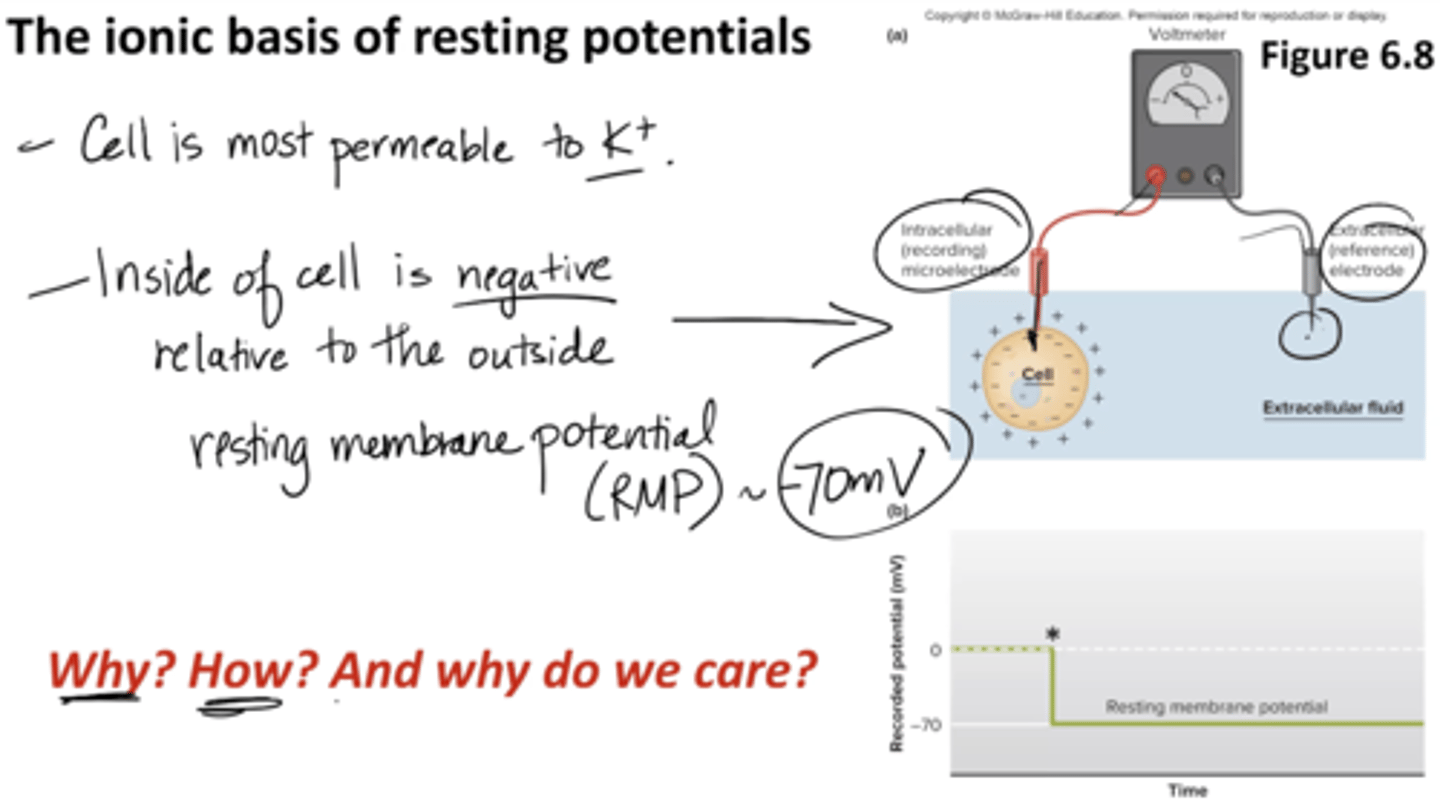
What is the ionic basis of resting potentials?
1. Cell is most permeable to potassium (K+)
2. Inside of cell is negative, relative to outside resting membrane potential (RMP –70mV)
- extracellular fluid has a lot of sodium but the cell is most permeable to potassium and it's negative
Why is the inside of the cell negative?
1. Na+/ K+ pump pumps 3 Na+ out for every 2 K+ that comes in
2. K+ passively flows with/down the gradient due to leak channels, creates (–) charge on membrane
*leak channels ions and voltage gated go down/gradient
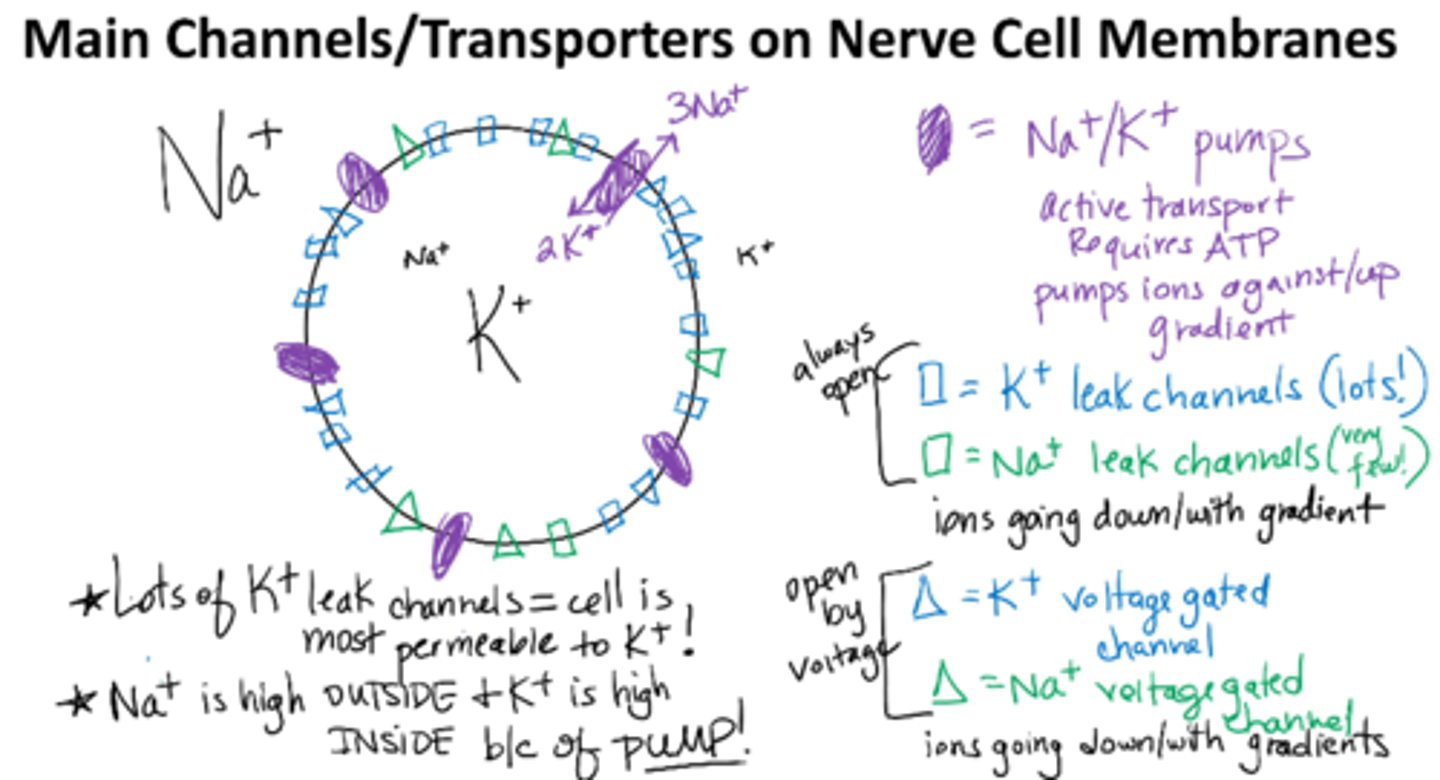
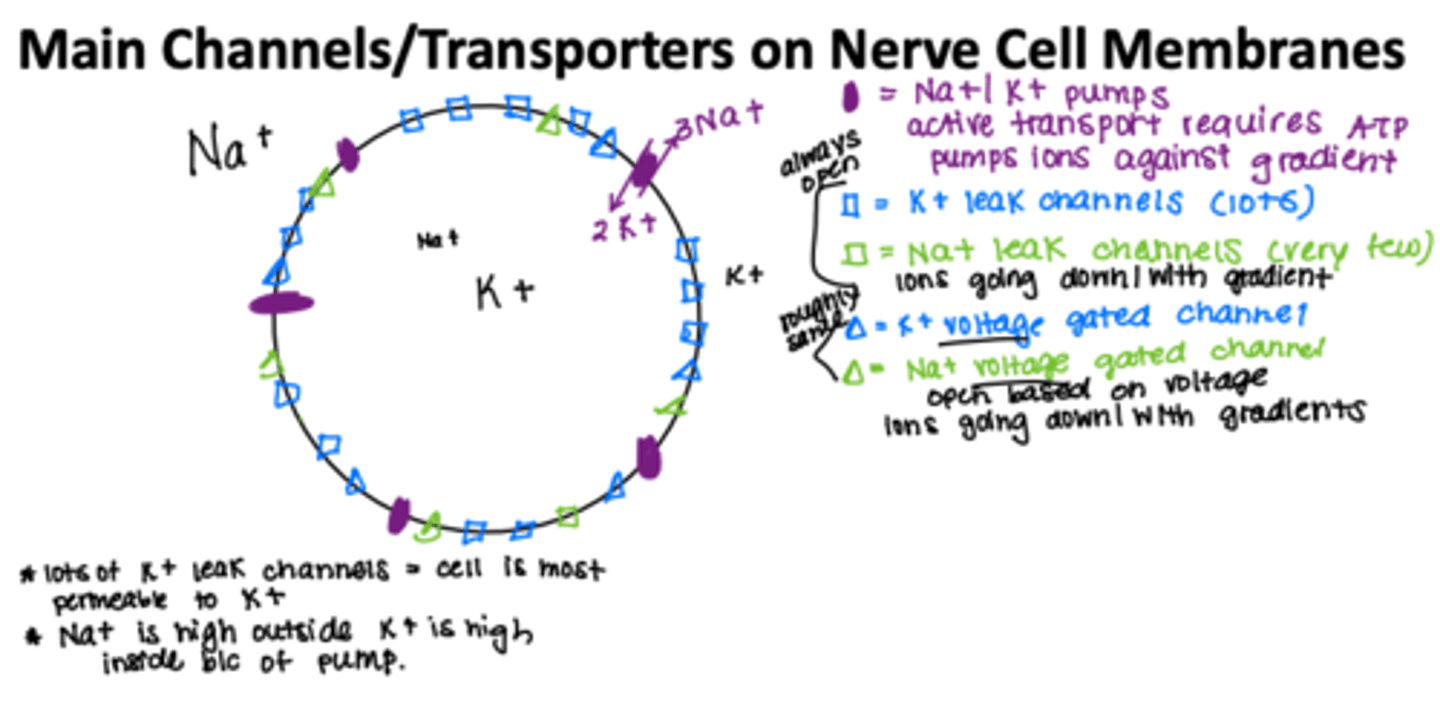
5 Main Channels/Transporters on Nerve Cell Membranes
-Na/K Pump
-Na leak channels very few
-K leak channels alot
--leak channels r always open !
-Na voltage gated channels
-K voltage gated channels
--open at different voltages
--open quicker
up the gradient
low to high concentration
aganist
down the gradient
with the gradient
high to low
Na+/K+ pump
maintain low intracellular Na+ concentration and high intracellular K+ concentration by active transport (requires ATP)
Intracellular fluid → rich in potassium
Extracellular fluid → rich in sodium and chloride
A neuron at rest has a resting potential of -70mV. You put this neuron in a solution where the concentration of potassium in the extracellular solution is much higher. What happens to the resting membrane potential?
it gets more positive (depolarizes)
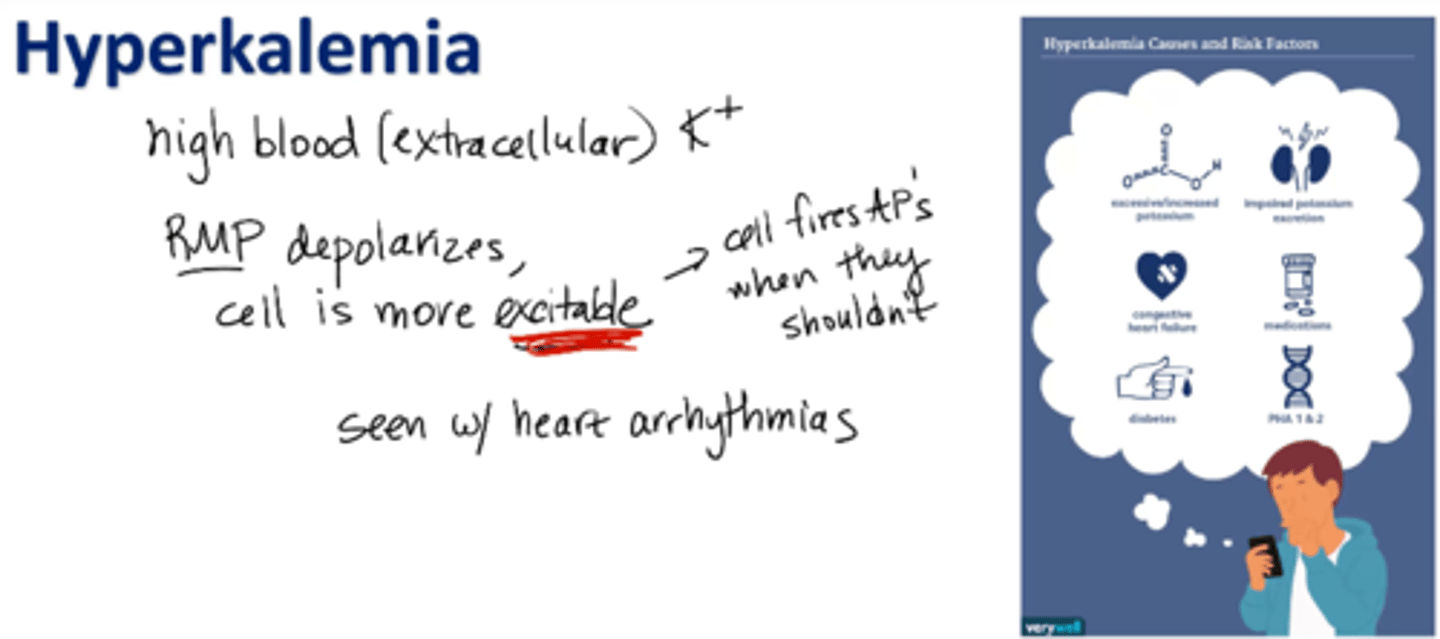
Hyperkalemia
high levels of potassium in blood (extracellular fluid)
RMP depolarizes, cell is more excitable (cell fires APs when they shouldn’t)
cardiac arrhythmias, shortness of breath, muscle weakness, etc.
Hypokalemia
deficient level of potassium in the blood
extracellular fluid is rich in
Na
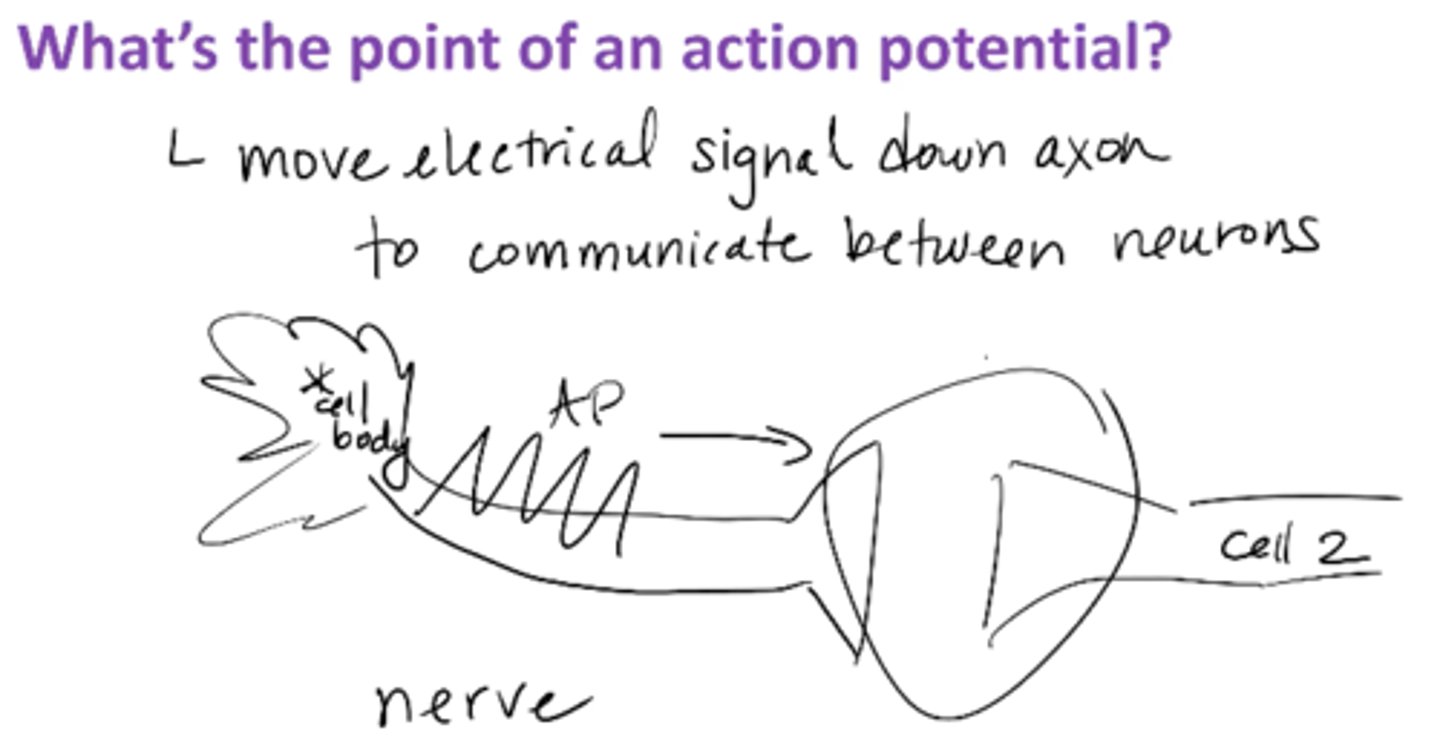
What's the point of an action potential?
moves electrical signal down axon to allow communication between neurons
COMMUNICATION
Nerve cells are most permeable to
K+
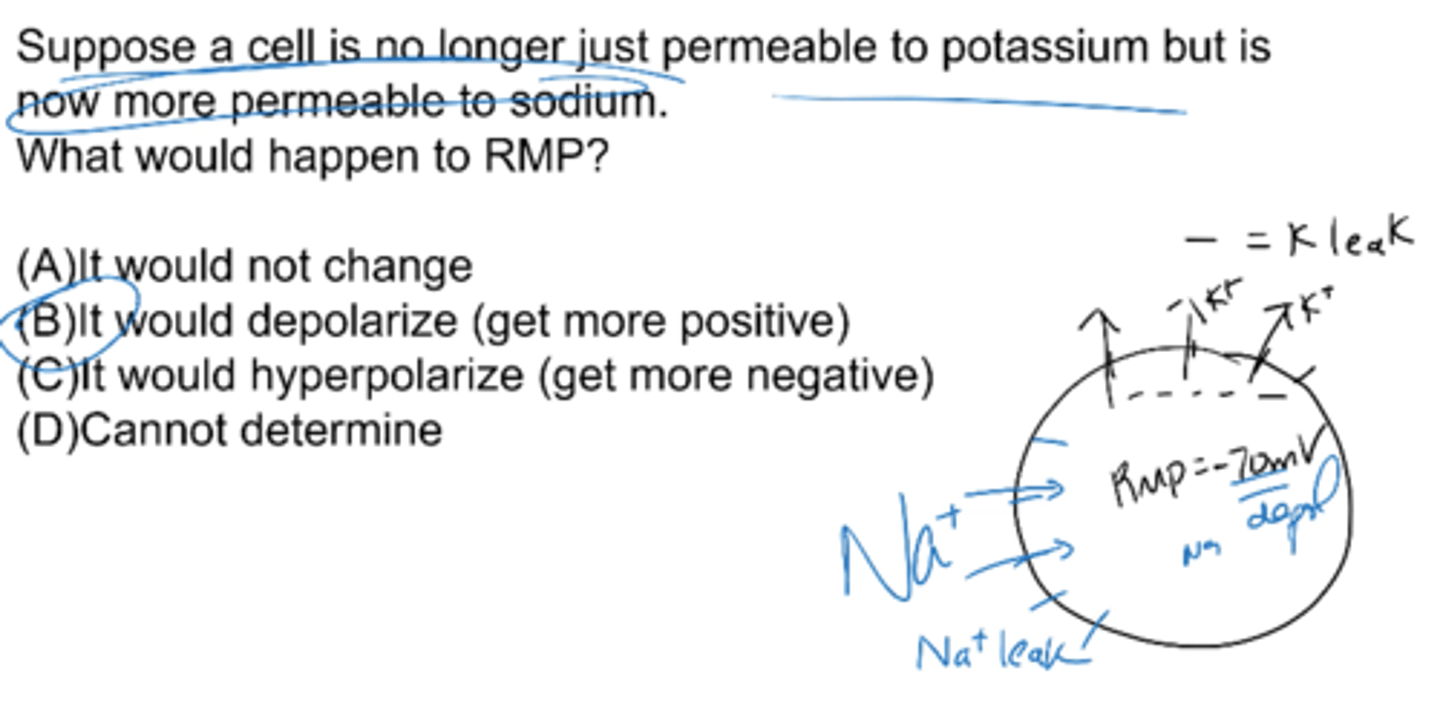
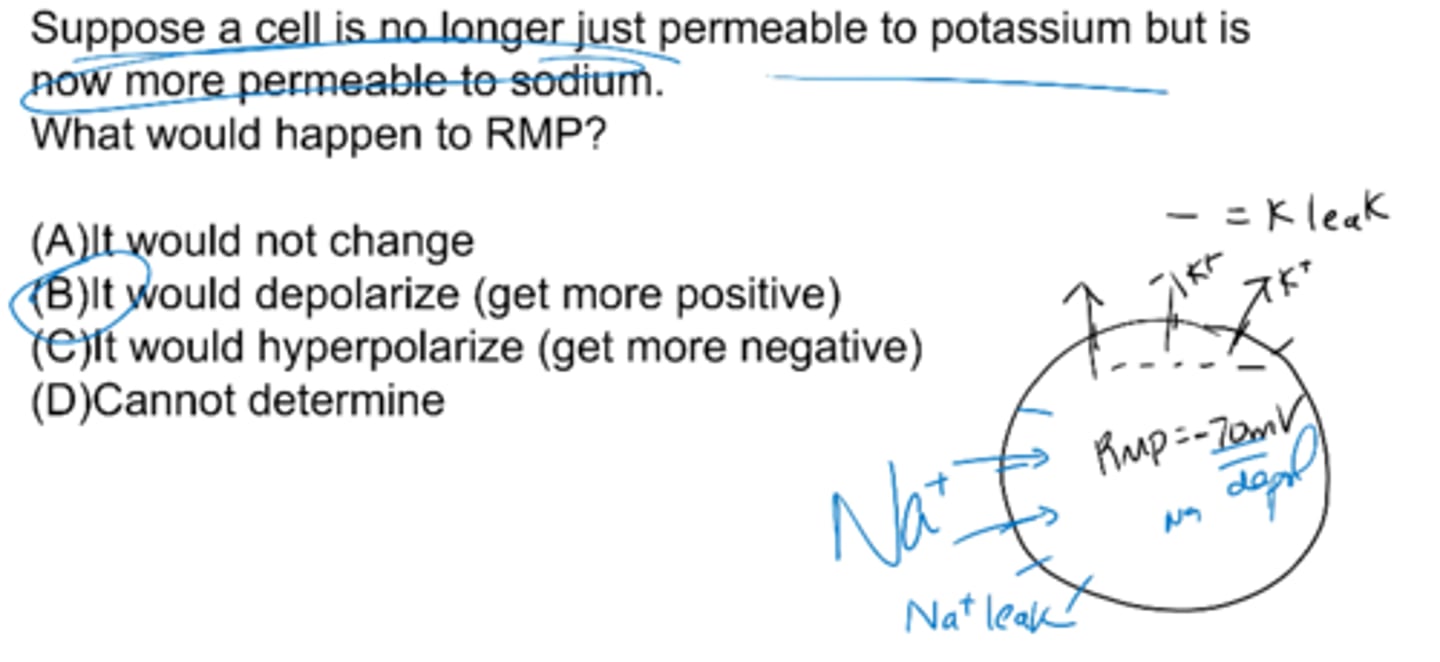
Suppose a cell is no longer just permeable to potassium but is now more permeable to sodium. What would happen to RMP?
it would depolarize (get more positive)
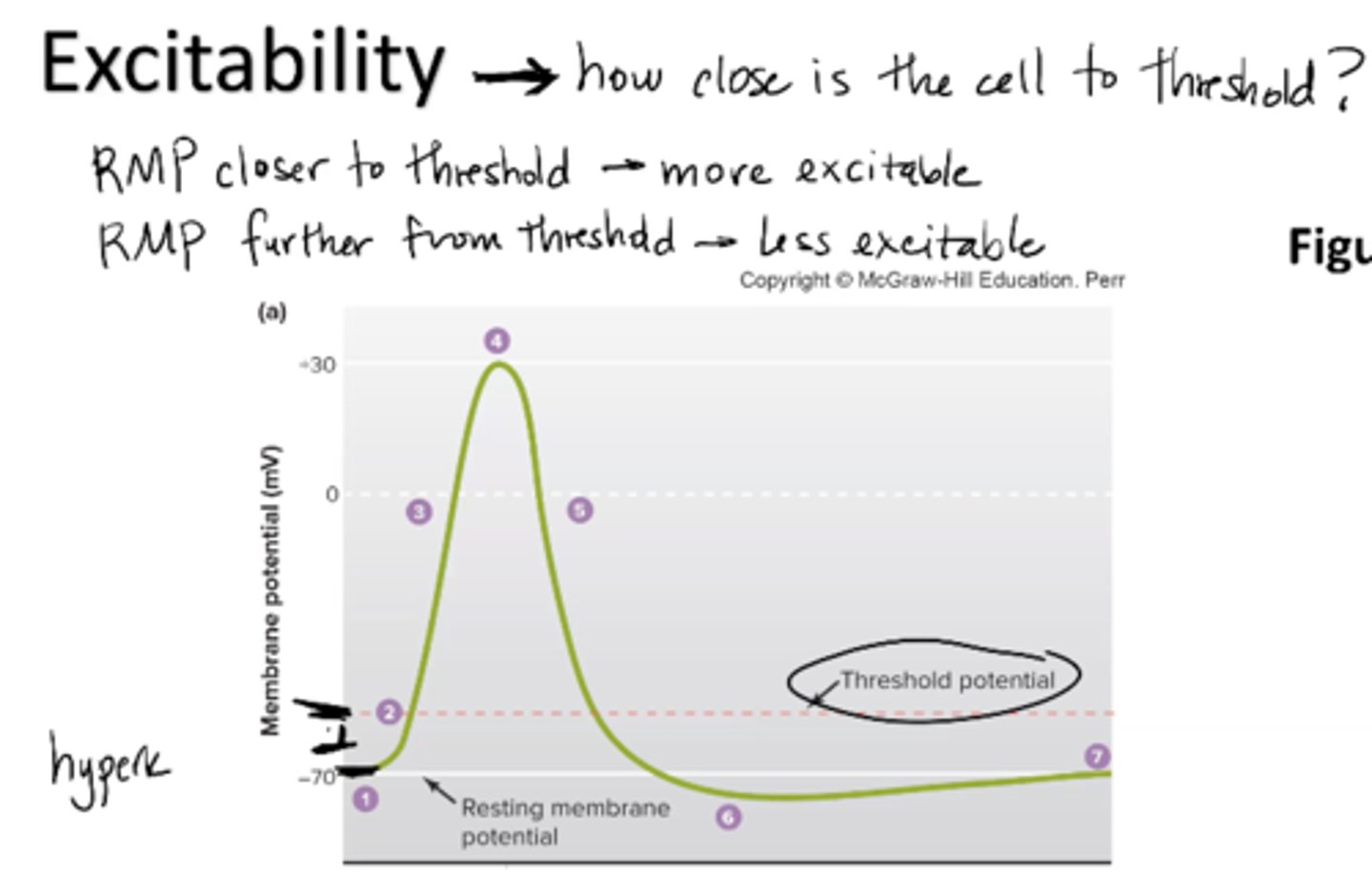
What is excitability?
ability to receive and respond to stimuli
how close is the cell to threshold?
RMP closer to threshold = more excitable
RMP further from threshold = less excitable
graded vs. action potentials
Graded (local) potentials – small local potentials with magnitudes that can vary and that die out
-can be hyper/depolarizing
-can sun
-decremental!?
Action potential (AP) – rapid, all-or-none change in membrane potential during which the membrane -
-always depolarizes
-mediated with voltage gated channels
-non-decremental
-dont sum
-indep of stimulus strength all or nothing
Moves electrical signal down axon to communicate between neurons
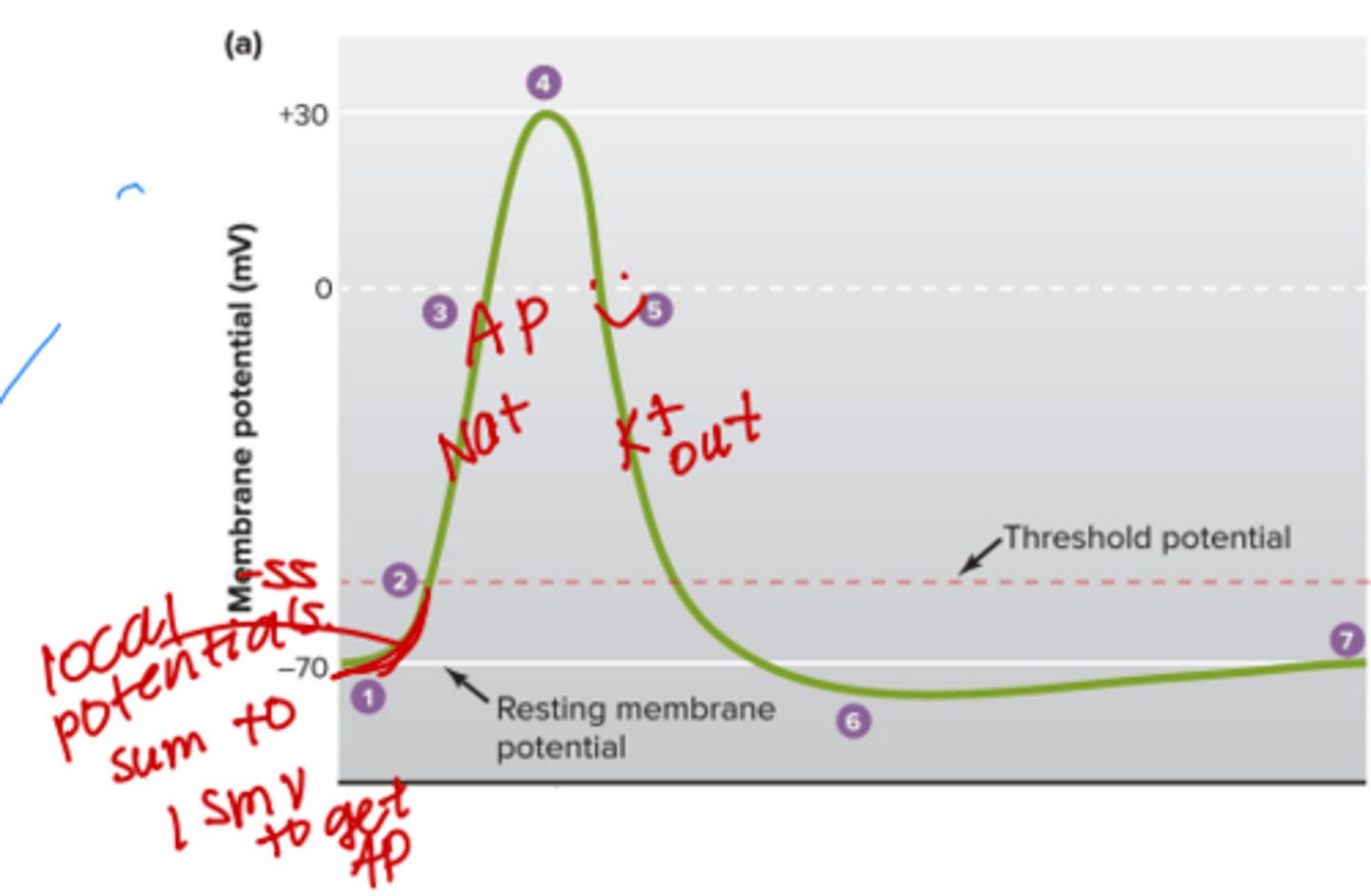
Where would the graded potentials be on this graph?
closer to the origin
Suppose a cell is no longer just permeable to potassium but is now more permeable to sodium. What would happen to RMP?
A) it would not change
B) it would depolarize (get more positive)
C) it would hyperpolarize (get more negative)
D) cannot determine
B) it would depolarize (get more positive)
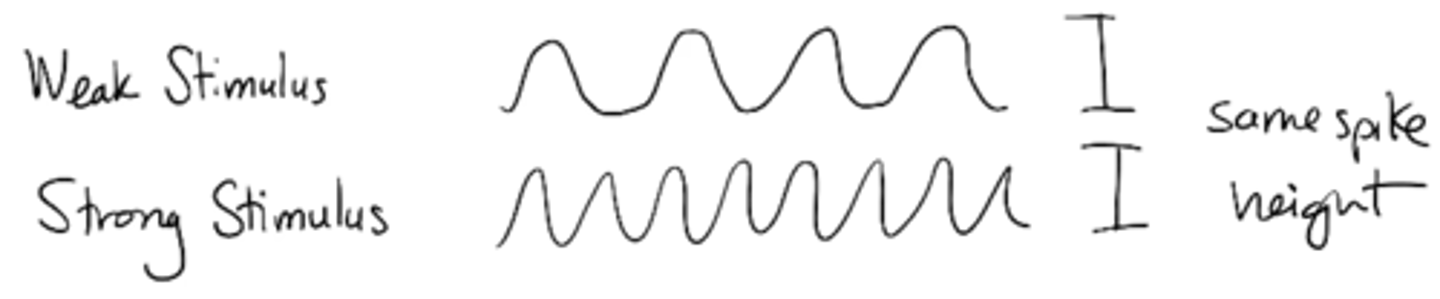
Explain how stimulus strength is coded by action potential frequency
Weak vs. strong stimulus would have same spike height but different frequency
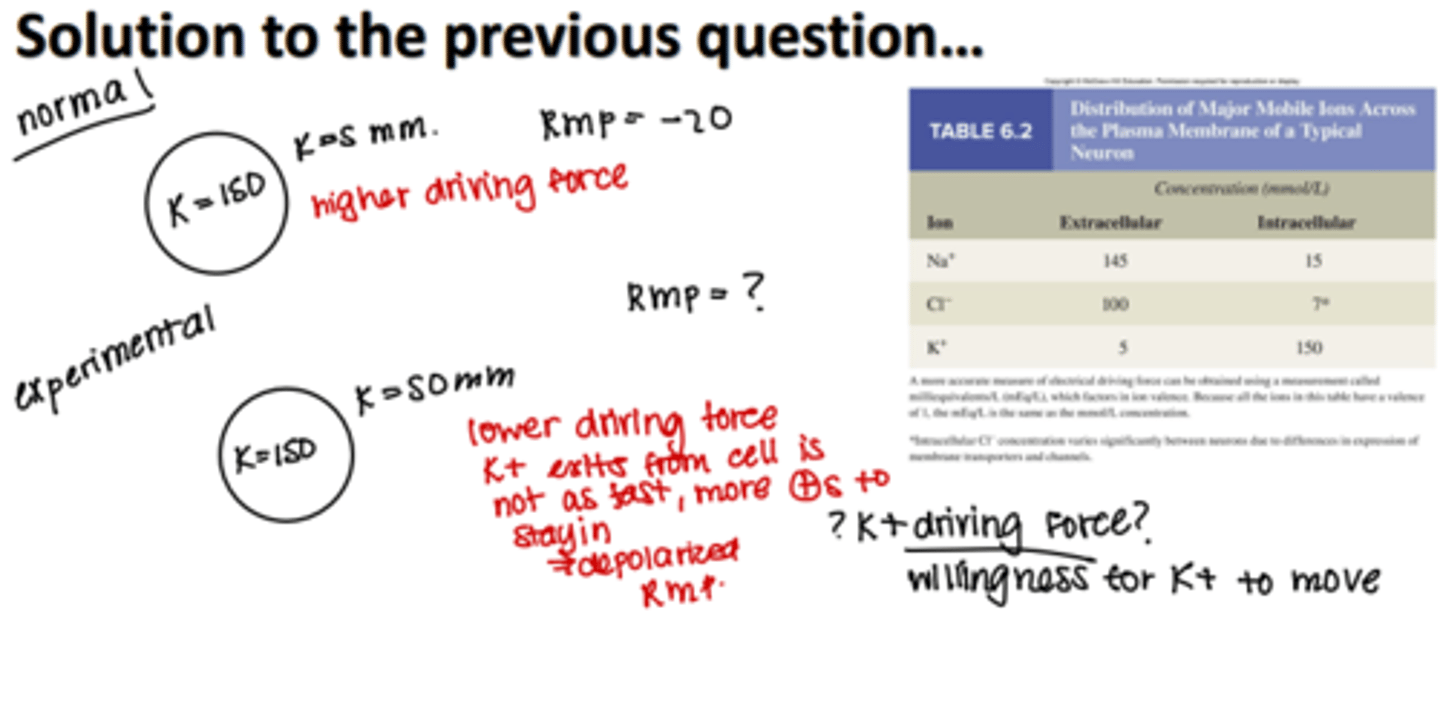
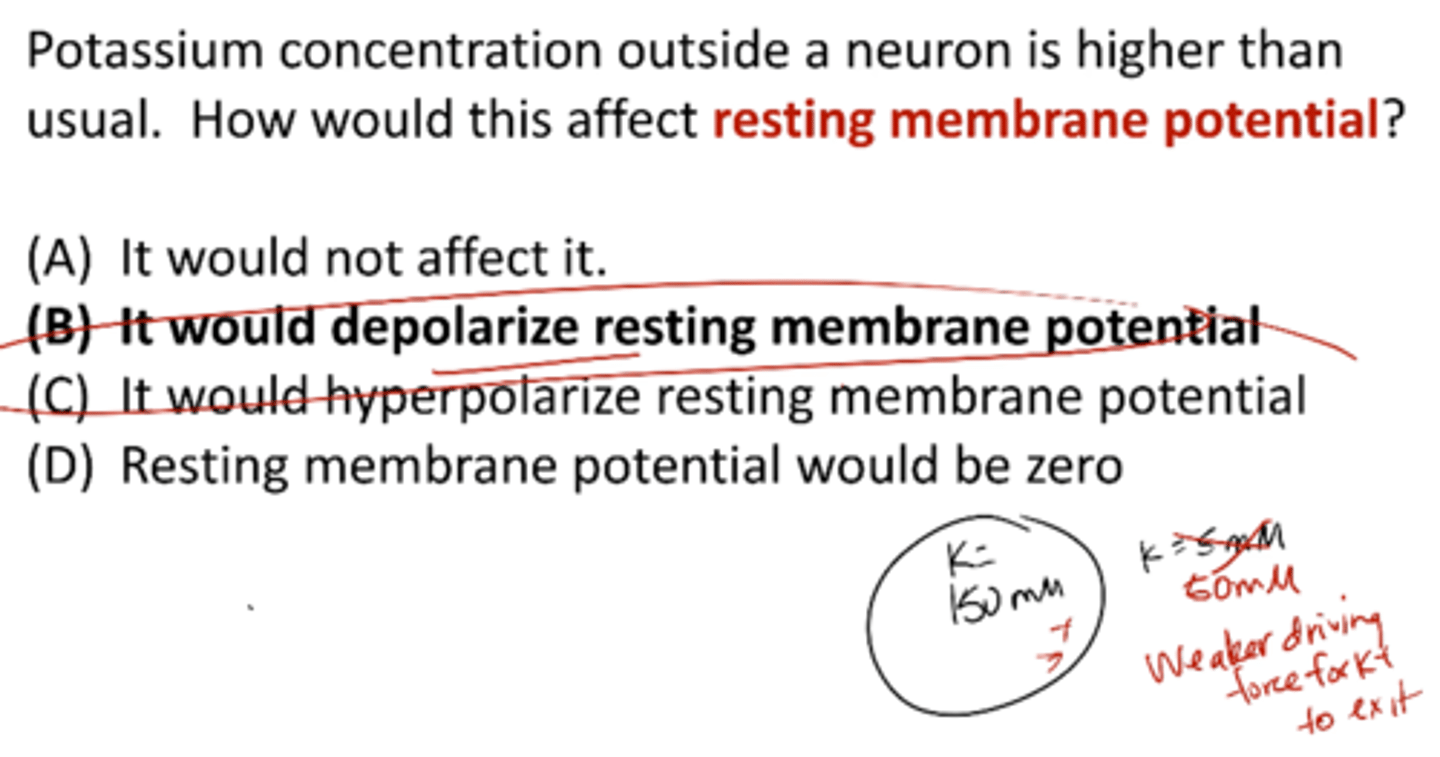
Potassium concentration outside a neuron is higher than usual. How would this affect resting membrane potential?
it would depolarize resting membrane potential
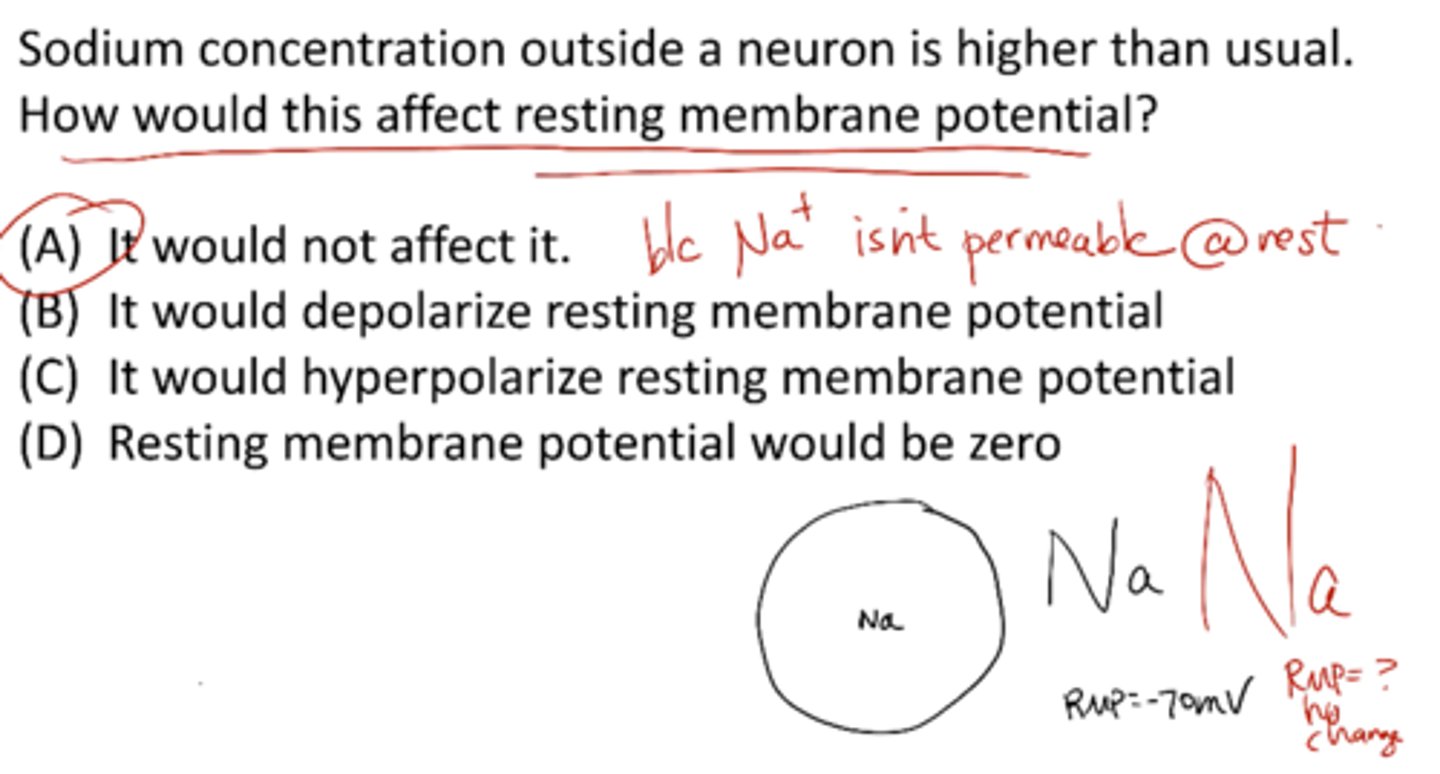
Sodium concentration outside a neuron is higher than usual. How would this affect resting membrane potential?
A) it would not affect it
B) it would depolarize RMP
C) it would hyperpolarize RMP
D) RMP would be zero
A) it would not affect it
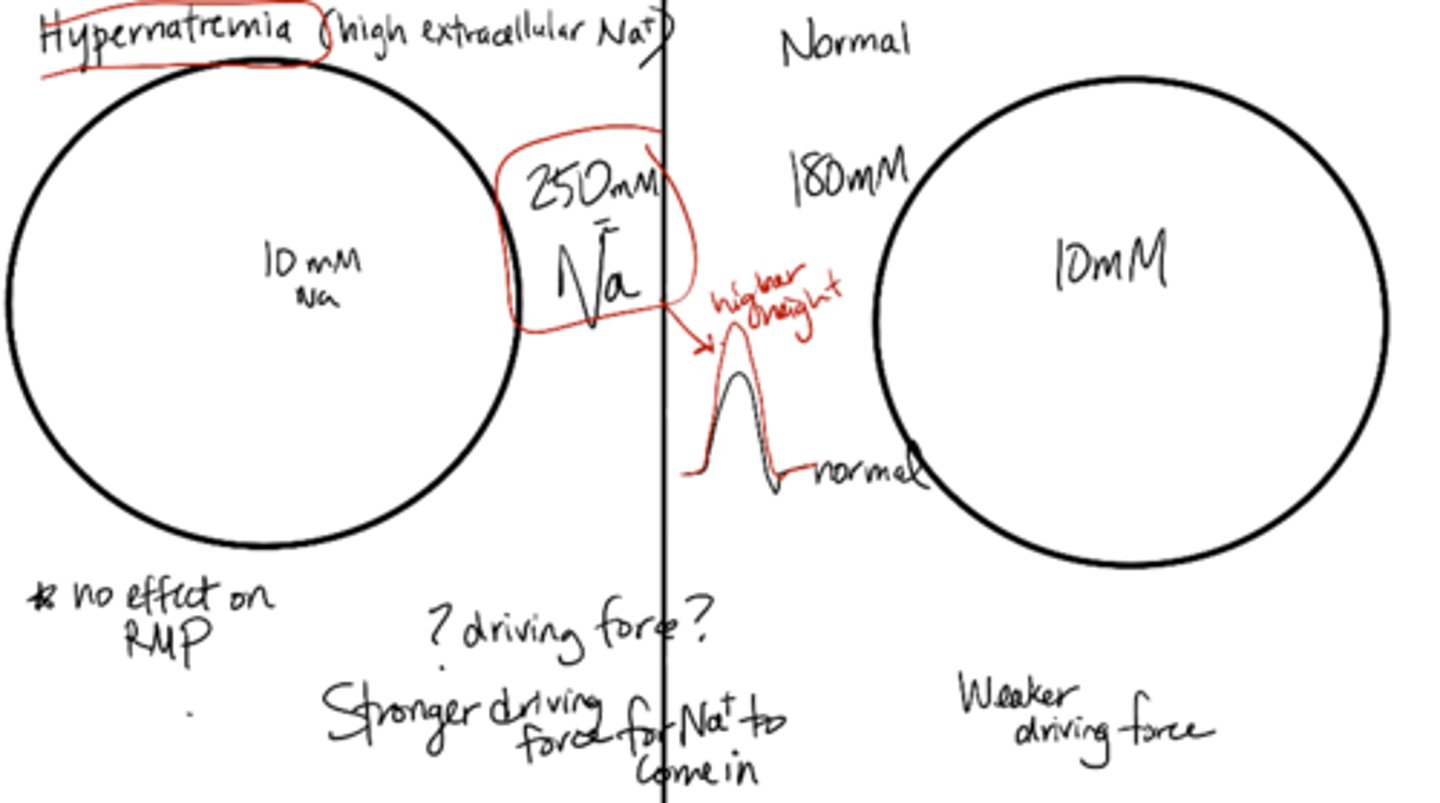
Hypernatremia
high extracellular sodium (no effect on RMP)
thirst, fatigue, restlessness (not drinking enough water)
Resting Membrane Potential (RMP) is dependent on:
changes in K+ (leak channels) (not Na+)
action potential spike height is dependent on:
changes in Na+ (VG Na+ channels) (not K+)
b/c of voltage gated channels opening when reaching spike height
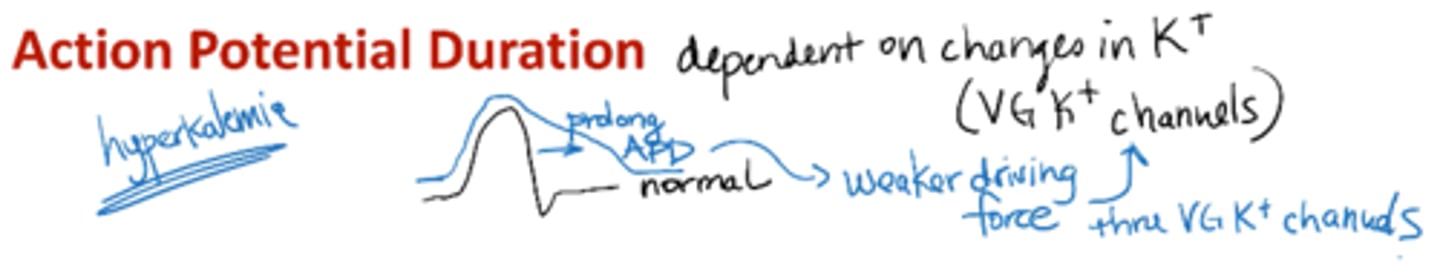
action potential duration is dependent on:
changes in K+ (VG K+ channels)
b/c of K voltage channels
ex: hyperkalemia
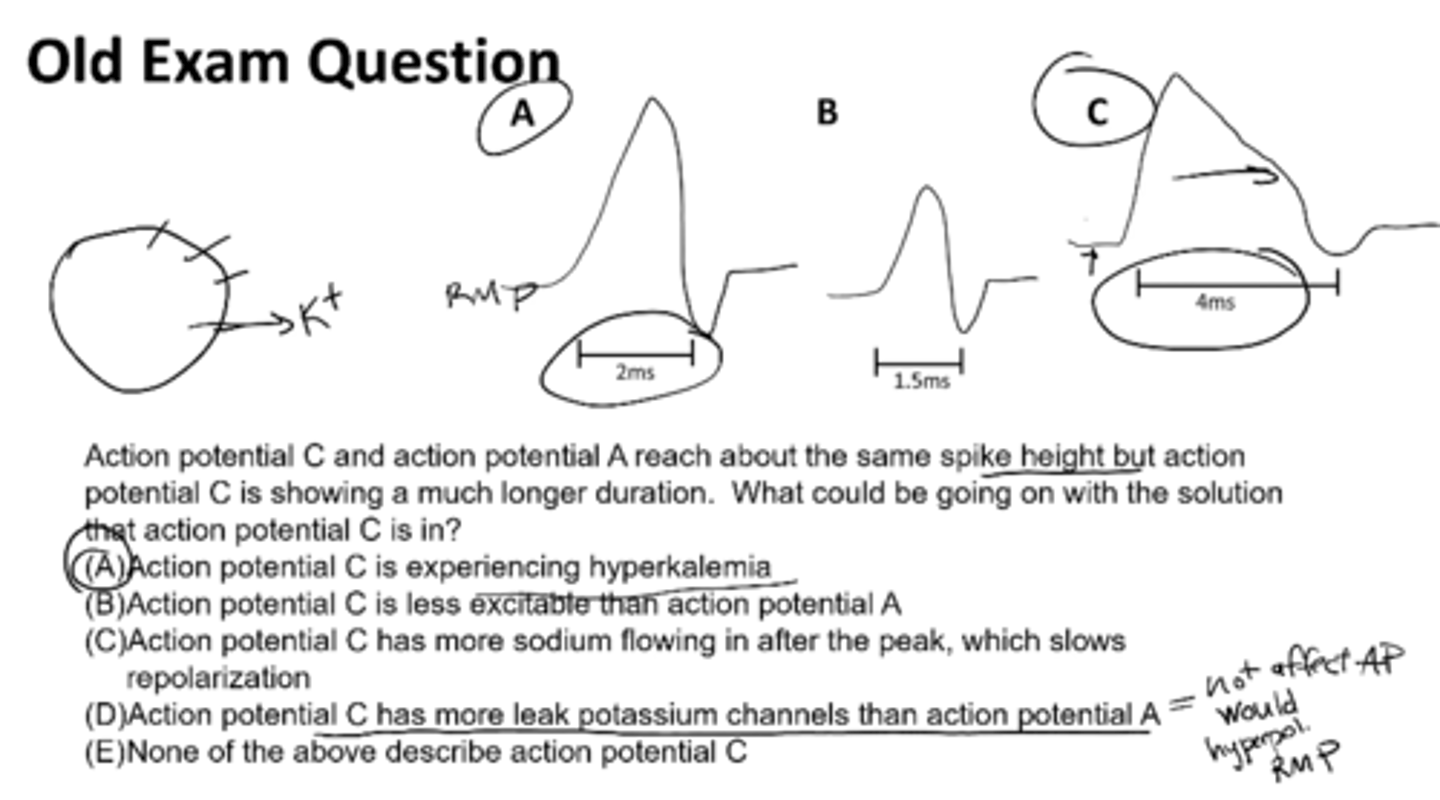
Action potential C and action potential A reach about the same spike height but action potential C is showing a much longer duration. What would be going on with the solution that action potential C is in?
action potential C is experiencing hyperkalemia
What is a synapse?
cell to cell junction (between neurons or a neuron and a muscle)
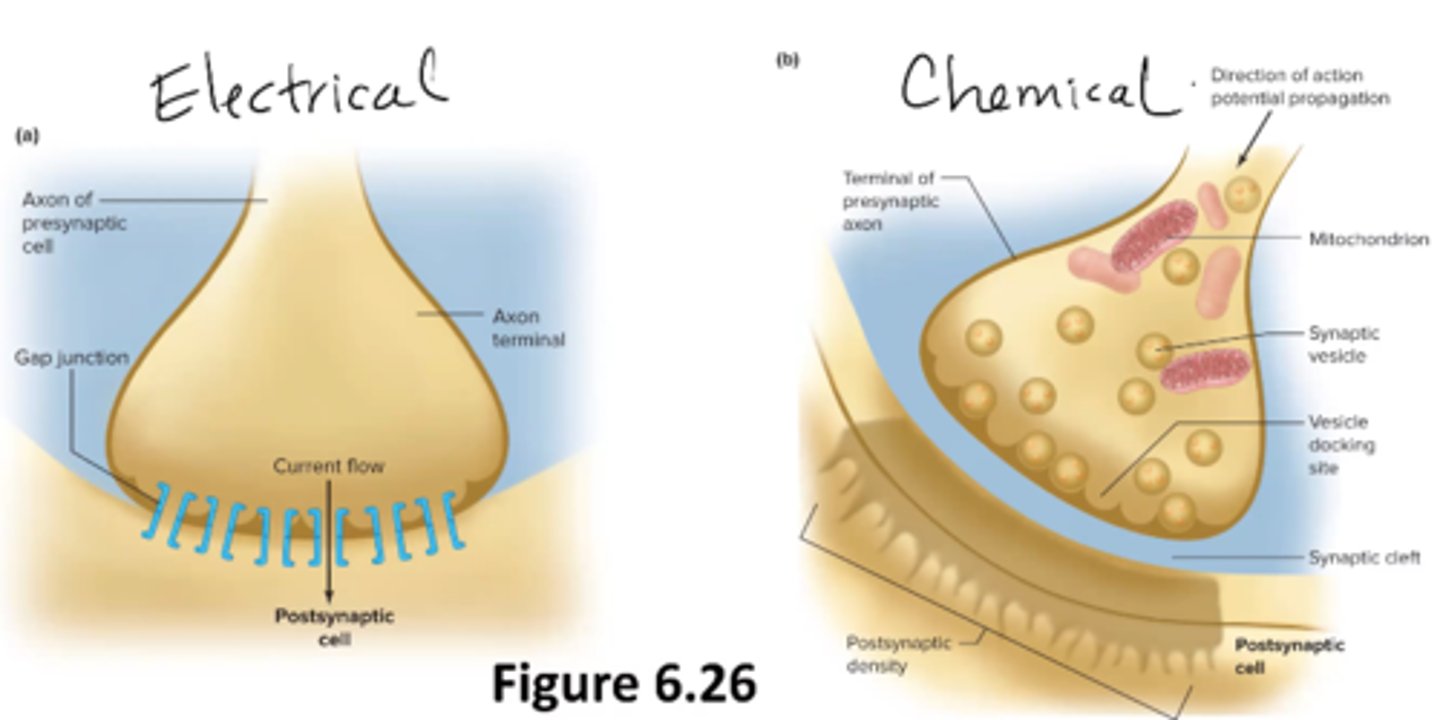
steps in a chemical synaptic transmission
1. AP propagates down pre-synaptic neuron
2. Voltage change from AP opens voltage-gated calcium channels, Ca2+ rushes into presynaptic terminal
3. Calcium helps synaptic vesicles fuse with presynaptic membrane, release of neurotransmitters into synaptic cleft
4. Neurotransmitters bind to receptor on postsynaptic cell
5. Response in postsynaptic cell (open/close channel, cell cascade)
6. Neurotransmitter removed from synaptic cleft
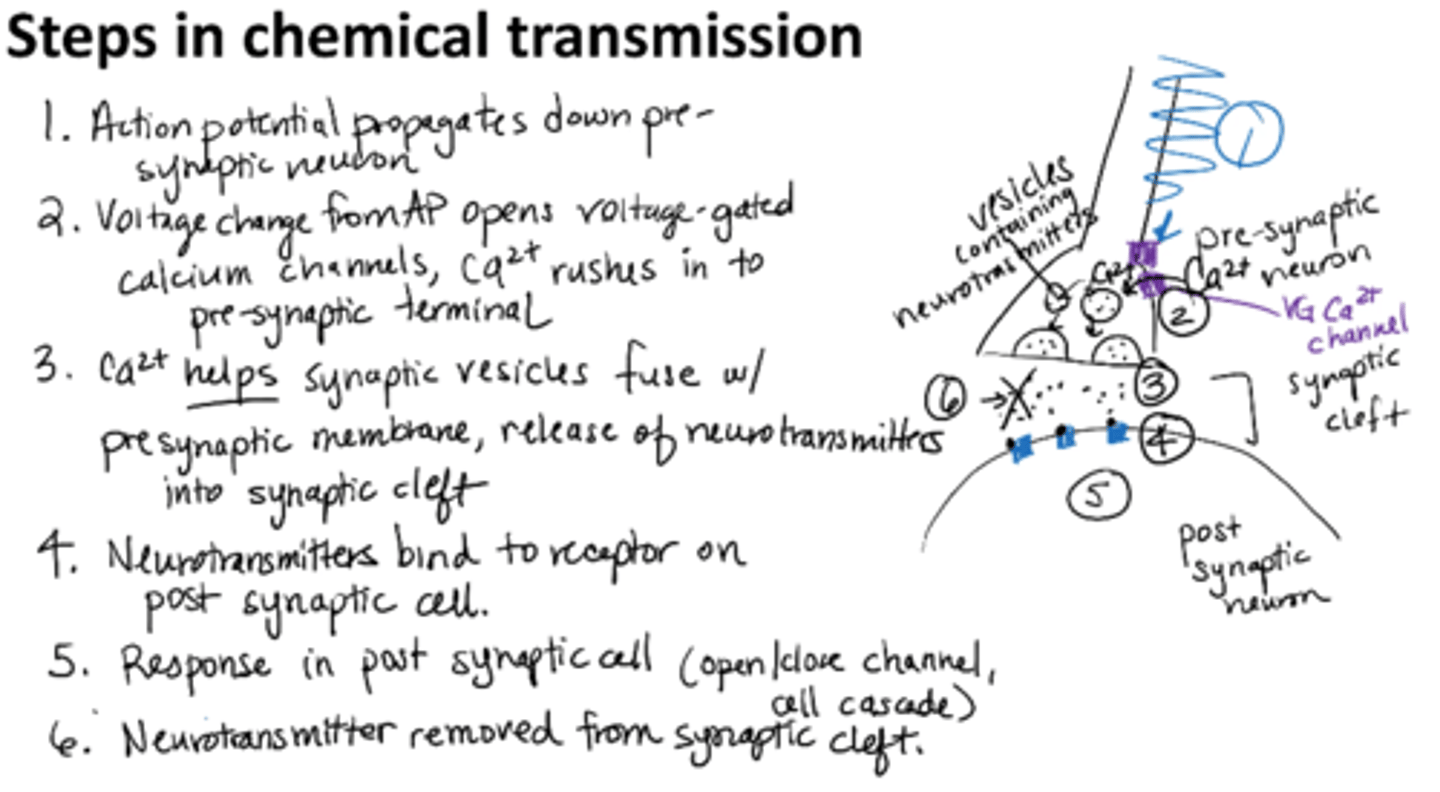
In a chemical synaptic transmission, what causes the calcium to come into the pre-synaptic neuron?
A) Na+ entry from pre-synaptic AP
B) vesicle fusion into the pre-synaptic membrane
C) a local potential in the cell body of the pre-synaptic neuron
A) Na+ entry from pre-synaptic AP

What determines if a synapse is excitatory or inhibitory?
Think: charge on the ion, concentration gradient
If a neurotransmitter binds and opens a channel that’s permeable to potassium, would this be an excitatory or inhibitory synapse?
Inhibitory bc K+ exits (Ks leaving makes inside more negative)

Suppose a cell is no longer just permeable to potasium but is now more permeable to sodium. What happens to RMP?
It depolarizes
-lets more sodium in making it more positive
stimulus strength depends on
action potential frequency
weak or strong stimulus produces same height but frequency is higher in stronger stimulus
potassium concentration higher outside of the cell how does this affect RMP
depolarizes
sodium concentration outside a neuron is higher than usual how does this affect RMP
doesnt
b/c theyre are few sodium leak channels so cell remains neutral
Explain host-synaptic responses vary, depending on the receptor and which ions flow.
ionotropic → faster
metabotropic → slower, long-lasting changes
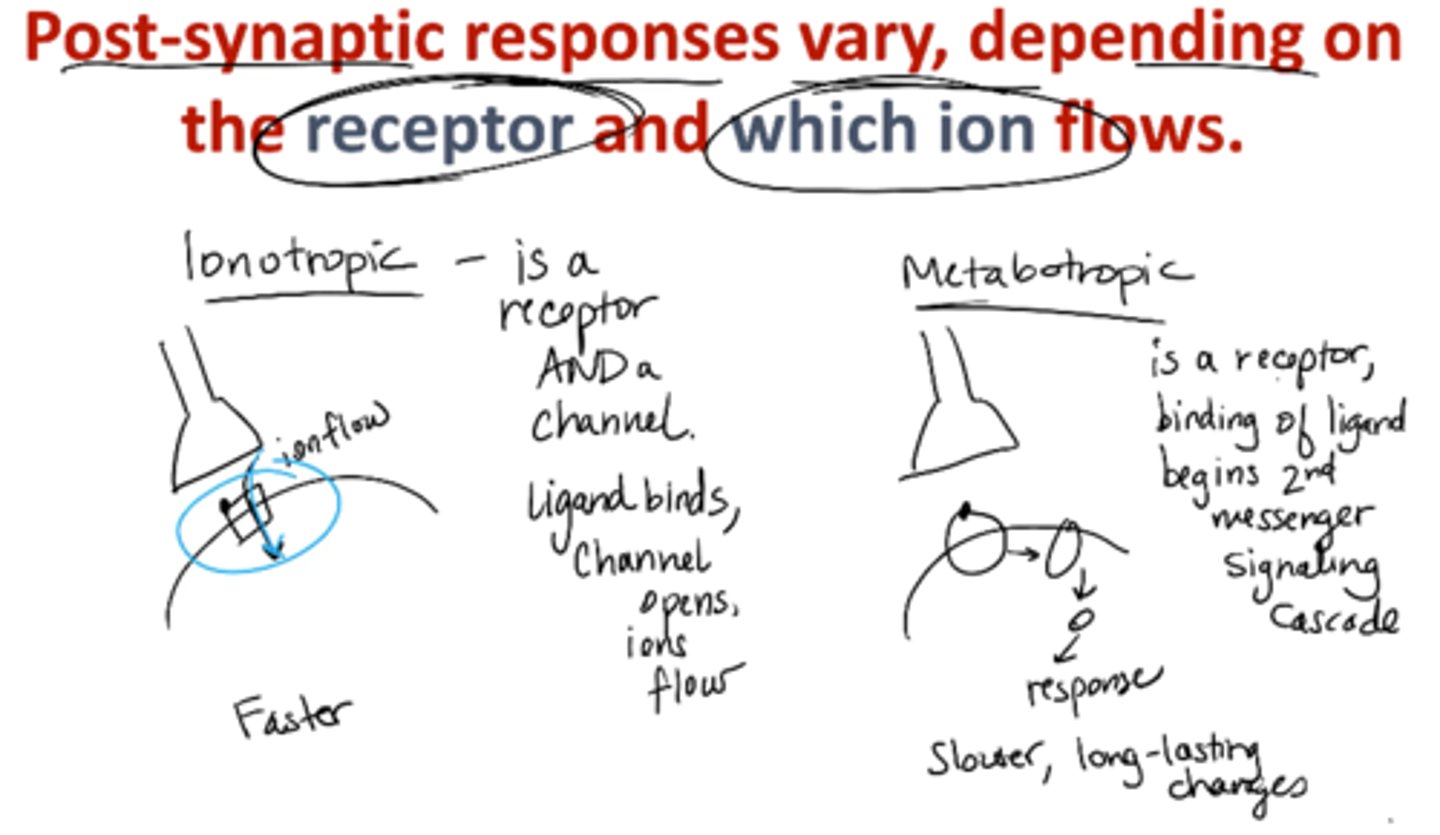
ionotropic receptors
receptor AND ligand-gated ion channel
Ligand binds, channel opens, ions flow
Faster
metabotropic receptors
receptors that act through a 2nd messenger system
binding of ligand begins 2nd messenger signaling cascade
slower, long-lasting changes
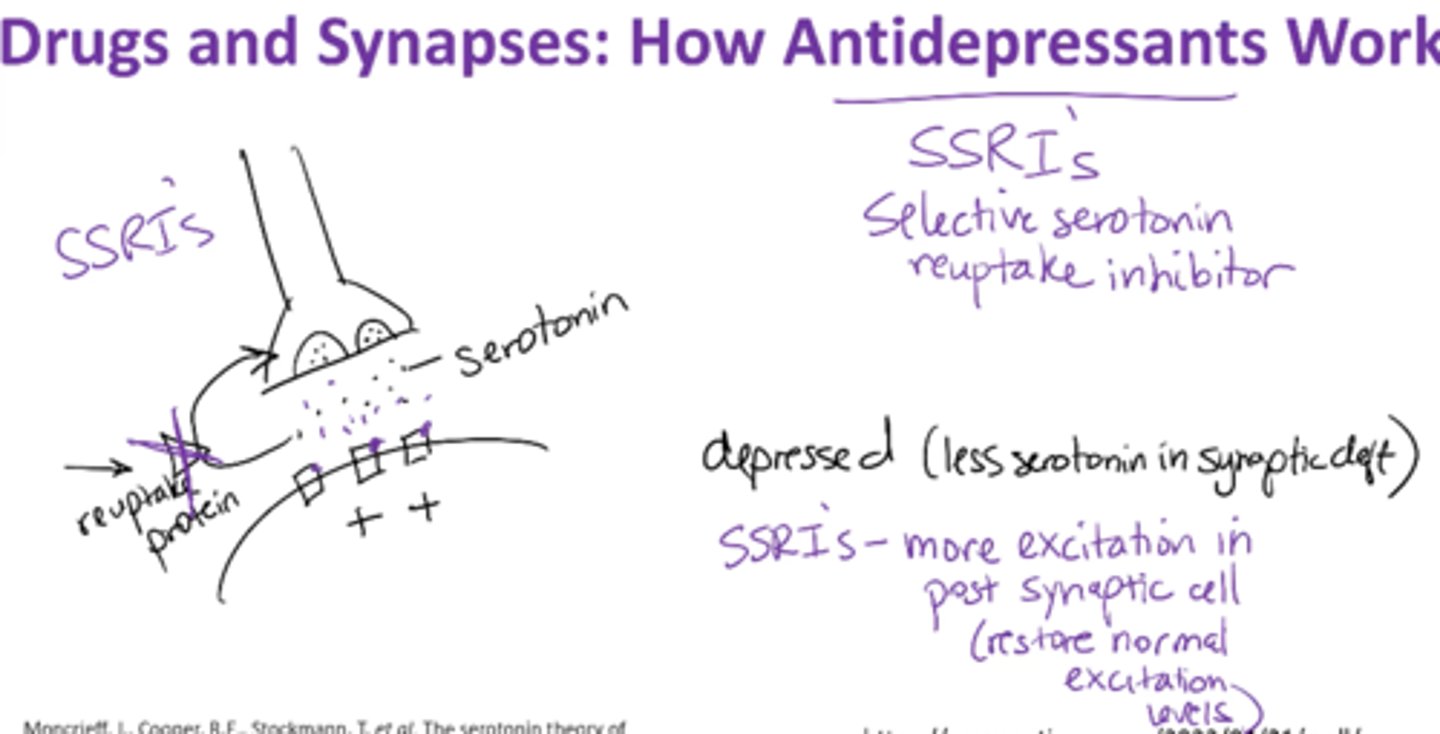
How do antidepressants work?
block reuptake of serotonin and norepinephrine
Depression → less serotonin in synaptic cleft
Selective serotonin reuptake inhibitor (SSRIs) –
-blocks serotonin inhibitor
-more excitation in postsynaptic cell;
-leaves more neurotransmitter in synaptic cleft
-restores normal excitation levels
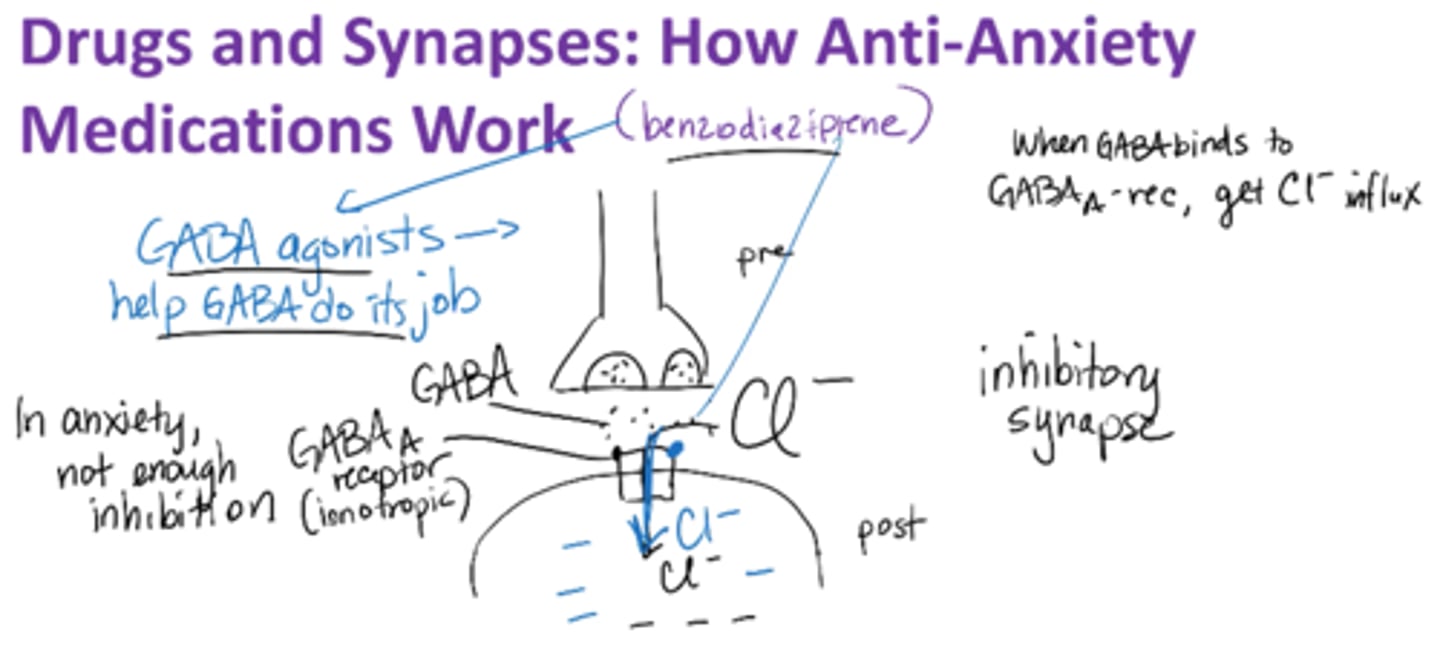
How do anti-anxiety medications work?
GABA agonist
promotes activity of GABA (Gamma-Aminobutyric Acid) which inhibits certain brain neurons, thus creates a calming effect
Anxiety → not enough inhibition !!
GABAA receptor → ionotropic
When GABA binds to GABAA-rec → gets Cl- influx
Anti-Anxiety meds are GABA receptor agonist
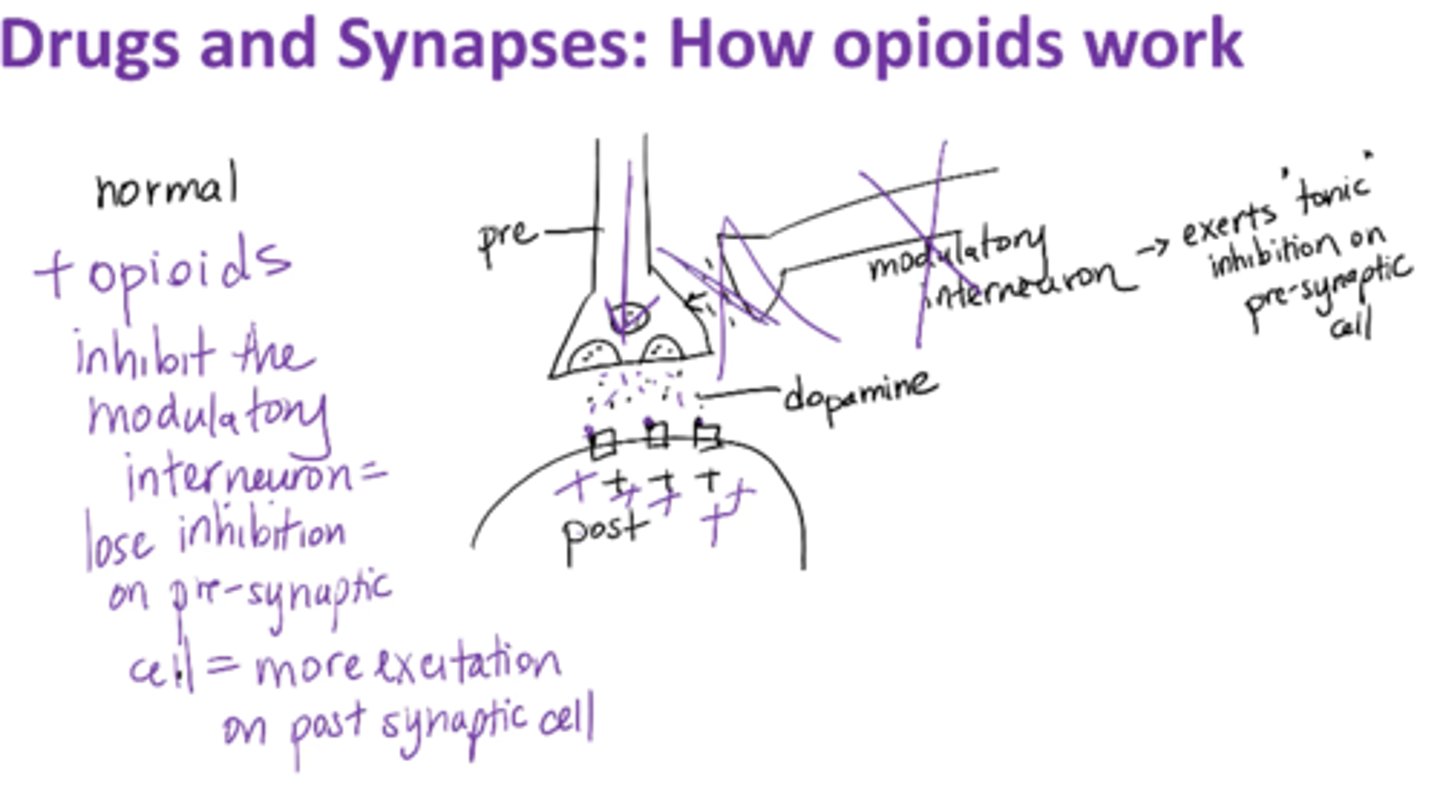
How do opiods work?
Modulatory interneuron – exerts “tonic (constant)” inhibition on presynaptic cell
Opioids inhibit the modulatory interneuron → lose inhibition on presynaptic cell → more excitation on post-synaptic cell
post-synaptic response is excitatory (metabotropic)
Narcan = antagonist
neurotransmitter is dopamine
how does cannabis work
endocanobinoids are endogenous( we make them)
-they are neuromodulators
-THC in weed binds to CB1 receptor and has dimming effect which slows your responses
-dopamine is neurotransmitter
What do our senses do?
change external information into graded/local/receptor potentials which eventually elicit action potentials
What are the 2 types of sensory receptors? Give examples of each and explain why smell is an exception.
Sensory receptor – neuron (smell)
Receptor cell – receives sensation, synapses on a neuron (sight, taste, hearing)
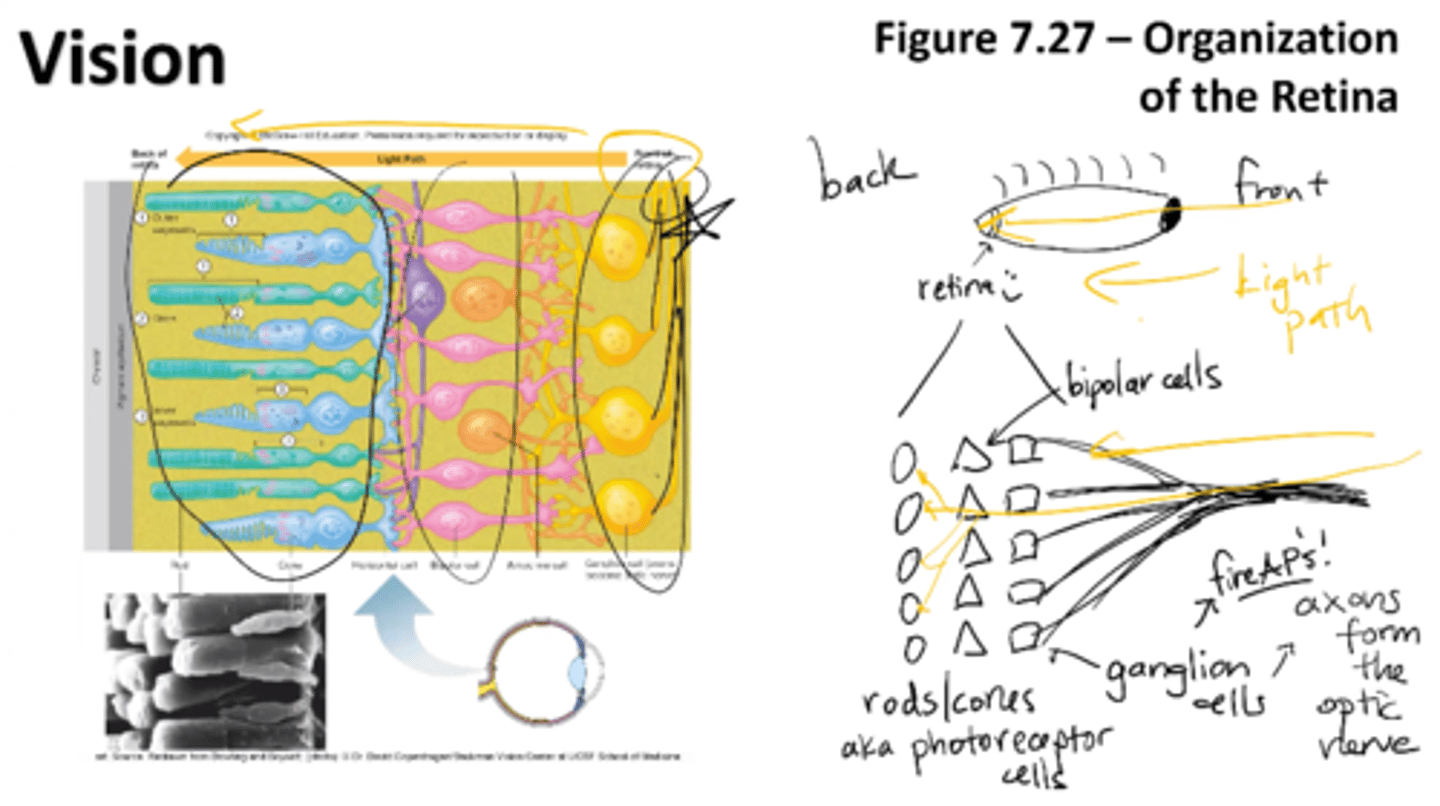
organization of the retina
Photoreceptor cells (rods/cones) lie at the back of the retina as well as ganglion cells (axons that form the optic nerve)
ganglion cells fire APs!
for light to reach the photoreceptors, it must pass layers of neurons involved in visual processing
rods/cons
bipolar cells
ganglion cells (axons from optic nerve)
RMP of -35mV
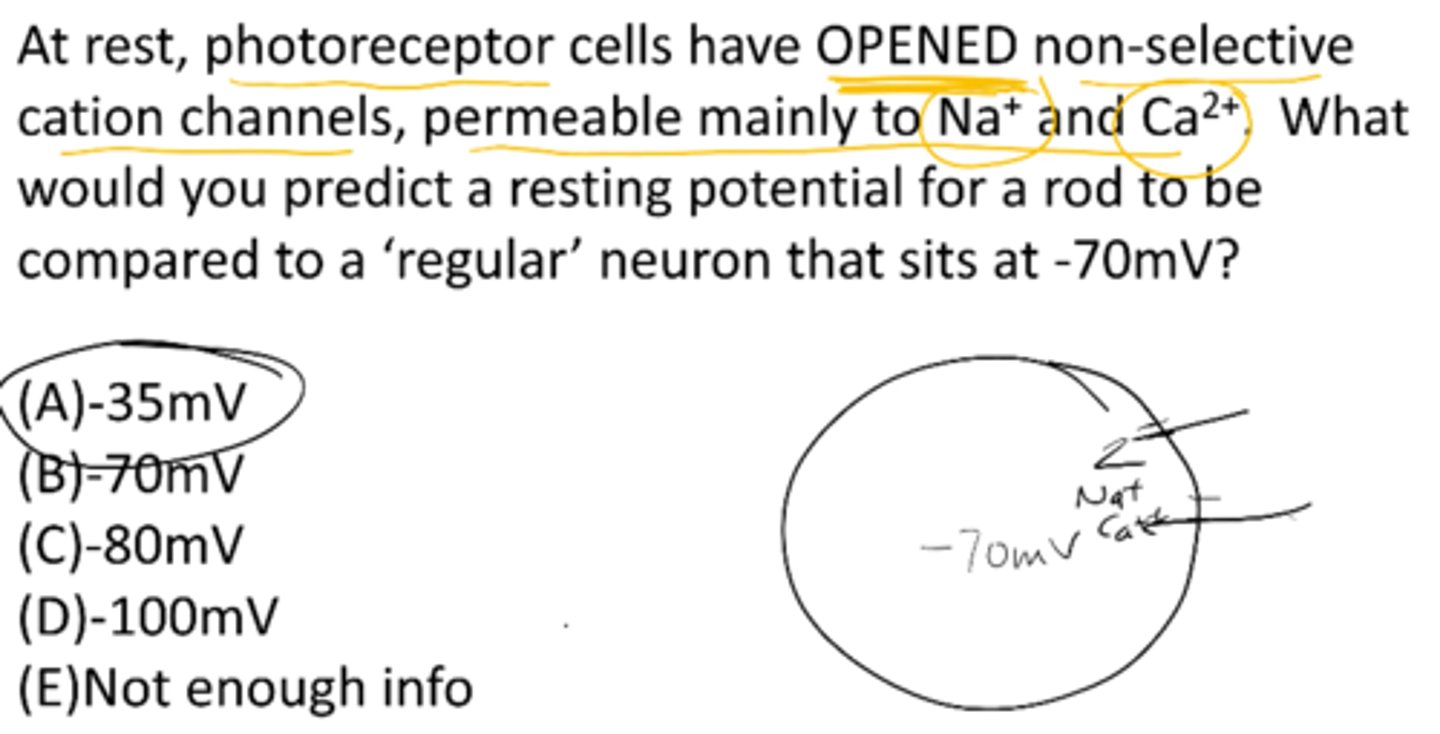
At rest, photoreceptor cells have OPENED non-selective cation channels, permeable mainly to Na+ and Ca+. What would you predict a resting membrane potential for a rod to be compared to a 'regular' neuron that sits at -70mV?
A) -35 mV
B) -70 mV
C) -80 mV
D) -100 mV
E) not enough info
A) -35 mV
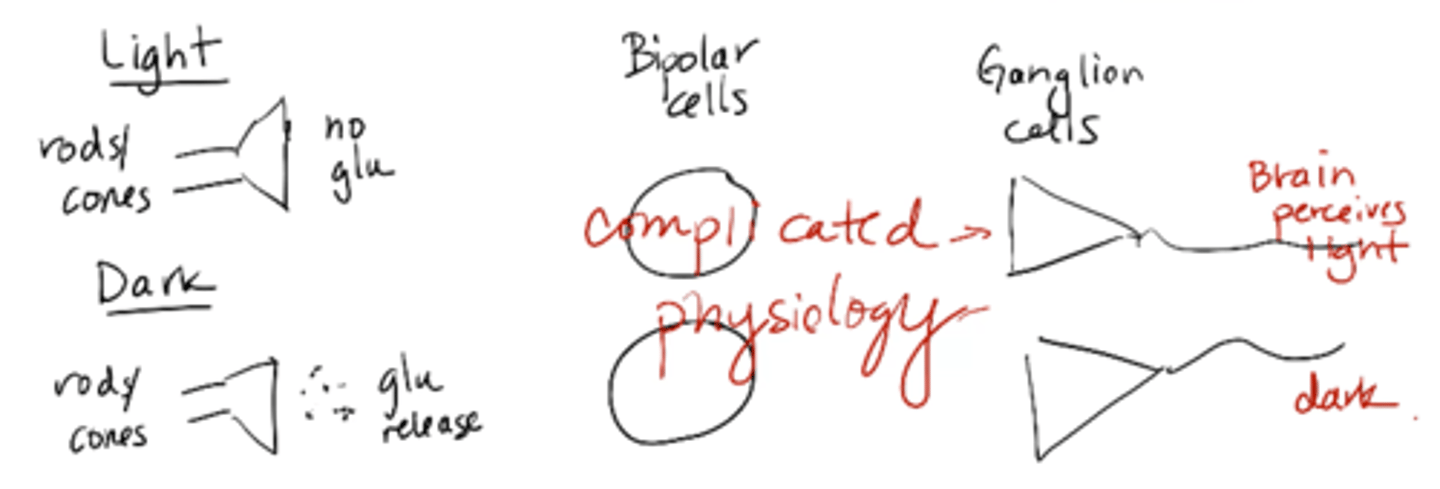
photoreceptor polarization
depolarized at rest (in the dark), hyperpolarized in the light
rhoposin
-key protein of rods/cons
-consists of retinal + opsin
is a membrane-bound protein
retinal – light-sensitive part of the photopigment
opsin- move in response to light

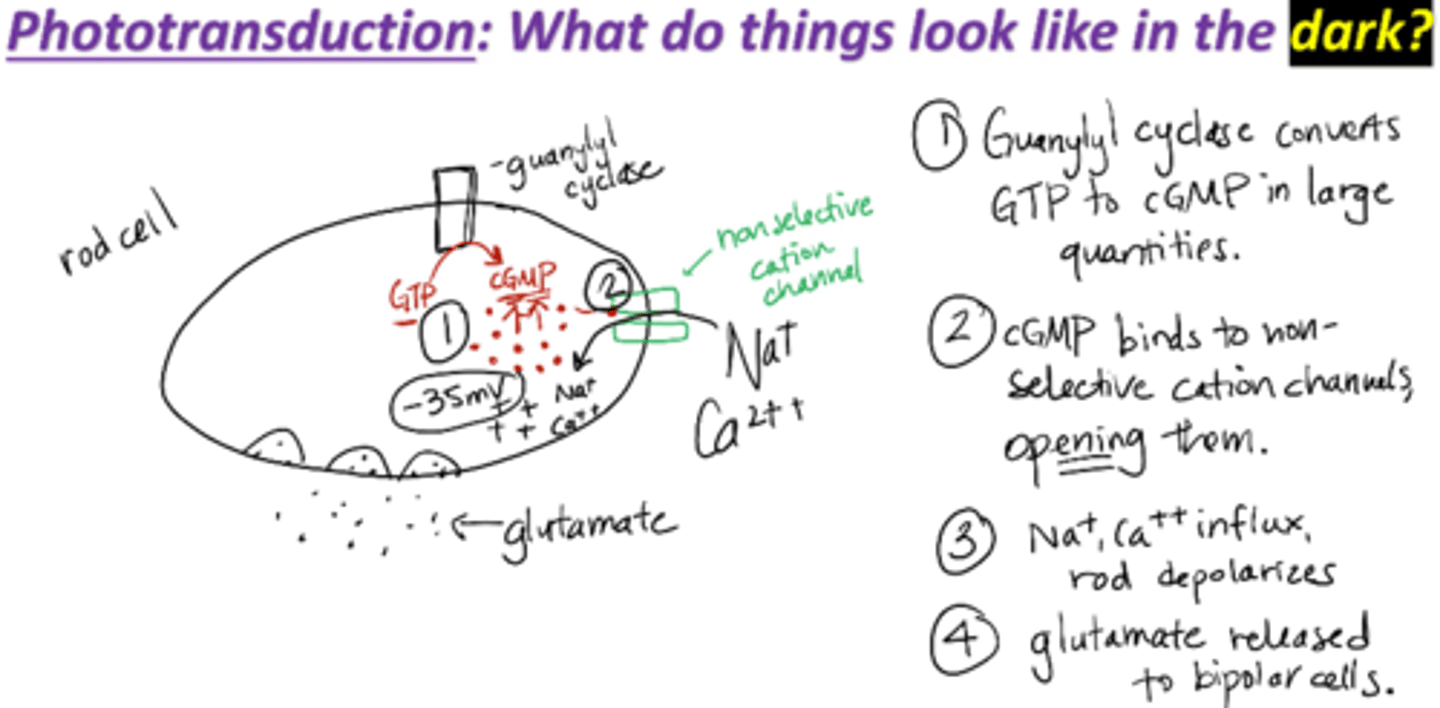
Phototransduction mechanism in the dark
1. Guanylyl cyclase converts GTP to cGMP in large quantities
2. cGMP binds to non-selective cation channels, opening them
3. Na+, Ca2+ influx through channel→ slight depolarization of rods
cGMP maintains outer segment ligand-gated cation channels in an open state, and a persistent influx of Na+ and Ca2+ occurs
Thus, in the dark, cGMP concentrations are high and the photoreceptor cell is maintained in a relatively depolarized state
4. Glutamate is released to bipolar cells
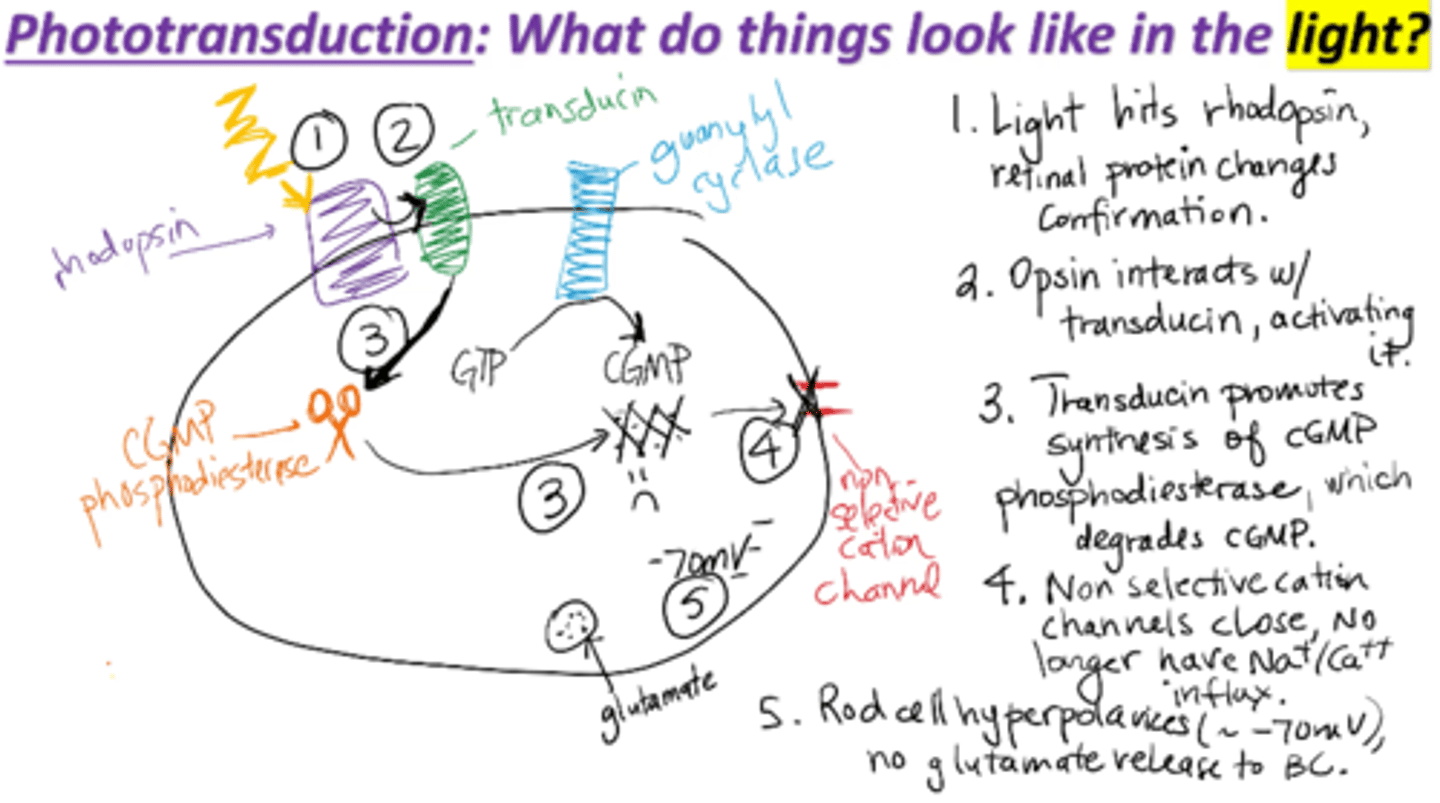
Phototransduction mechanism in the light
1. Light hits rhodopsin, retinal protein changes conformation
2. Opsin interacts with transducin, activating it
3. Transducin promotes synthesis of cGMP phosphosiesterase, which degrades cGMP
4. Non-selective cation channels close, no longer have Na+/Ca2+ influx
5. Rod cell hyperpolarizes (~70 mV), no glutamate release to bipolar cells
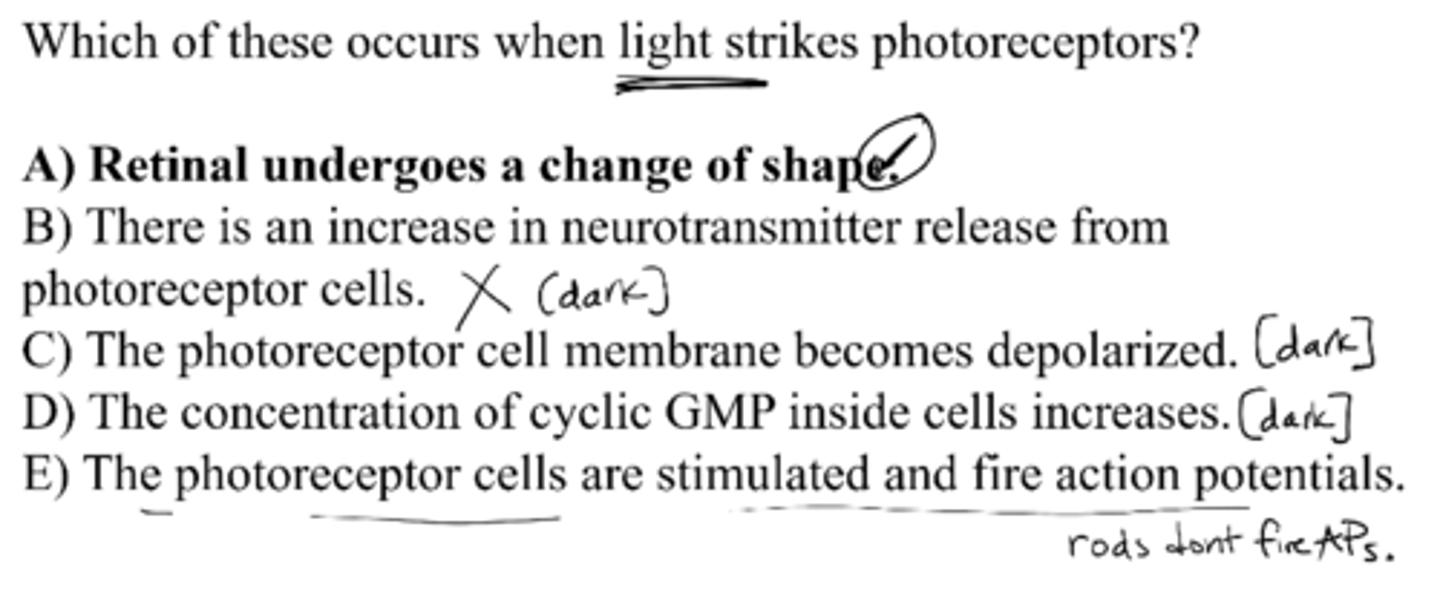
Which of these occurs when light strikes photoreceptors?
A) retinal undergoes a change of shape
B) there is an increase in neurotransmitter release from receptor cells
C) the receptor cell membrane becomes depolarized
D) the concentration of cyclic GMP inside cells increases
E) the photoreceptor cells are stimulated and fire action potentials
A) retinal undergoes a change of shape
Rods and cones are slightly depolarized and in the light they are hyperpolarized. Why does this matter in terms of being able to see?
picture
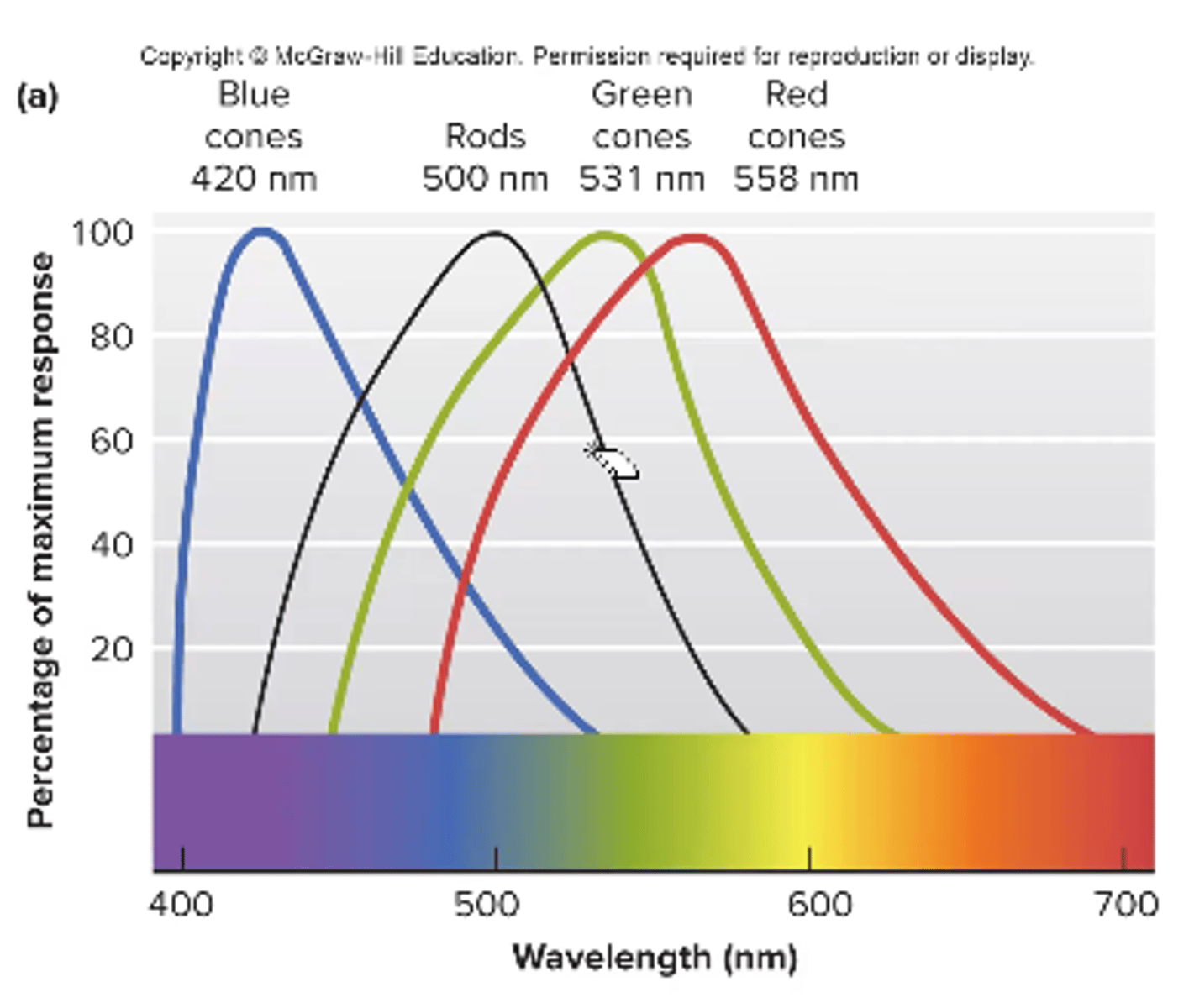
color vision/blindness
we have 3 types of cones that absorb light at different wavelengths
color blindness can occur as a result of missing 1 type of cone
red + green is most common
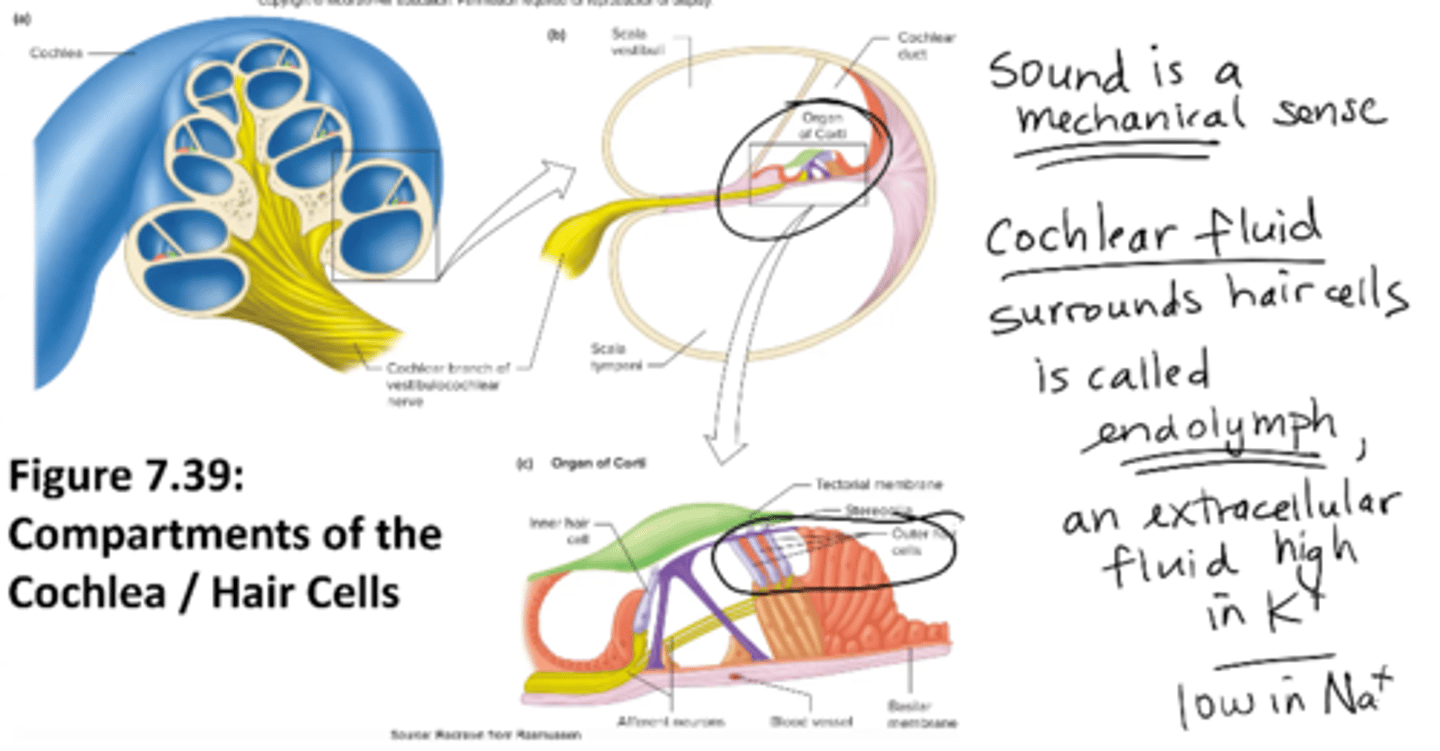
What is an endolymph?
cochlear fluid that surrounds hair cells
an extracellular fluid high in K+
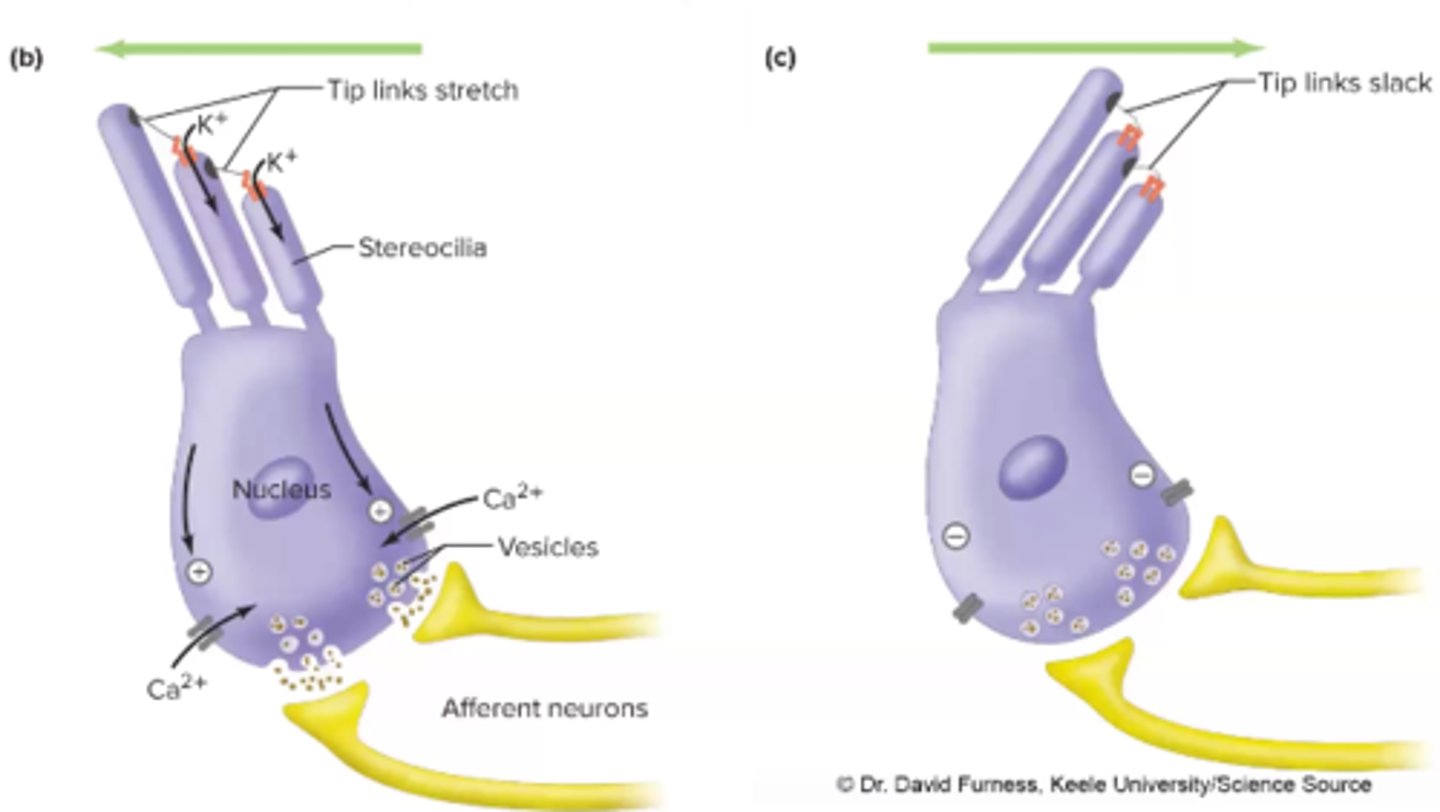
Tip links of stereocilia and Sound Production
move as a result of sound
-movement of the tip links open mechanically gated K+ ion channels
-K+ flows into hair cell and depolarizes it
-depolarization open VG Ca2+ channels ad Ca2+ flows into hair cell
-Ca2+ triggers neurotransmitter release (glutamate) and eventually elicits
AP on neighboring nerve cell --> HEARING
The hair cell in the ear is analogous to the ______ cell in the eye
rod and cone (both are receptor cells)
smell receptor
metabotropic (GOLF)
chemical messenger: odorants
emotional b/c they don't synapse on thalamus
What are the 4 main proteins of the sarcomere (muscle)?
Contractile proteins:
Actin (thin)
Myosin (thick)
Regulatory proteins
Troponin
Tropomyosin
if actin and myosin r interacting their must be force
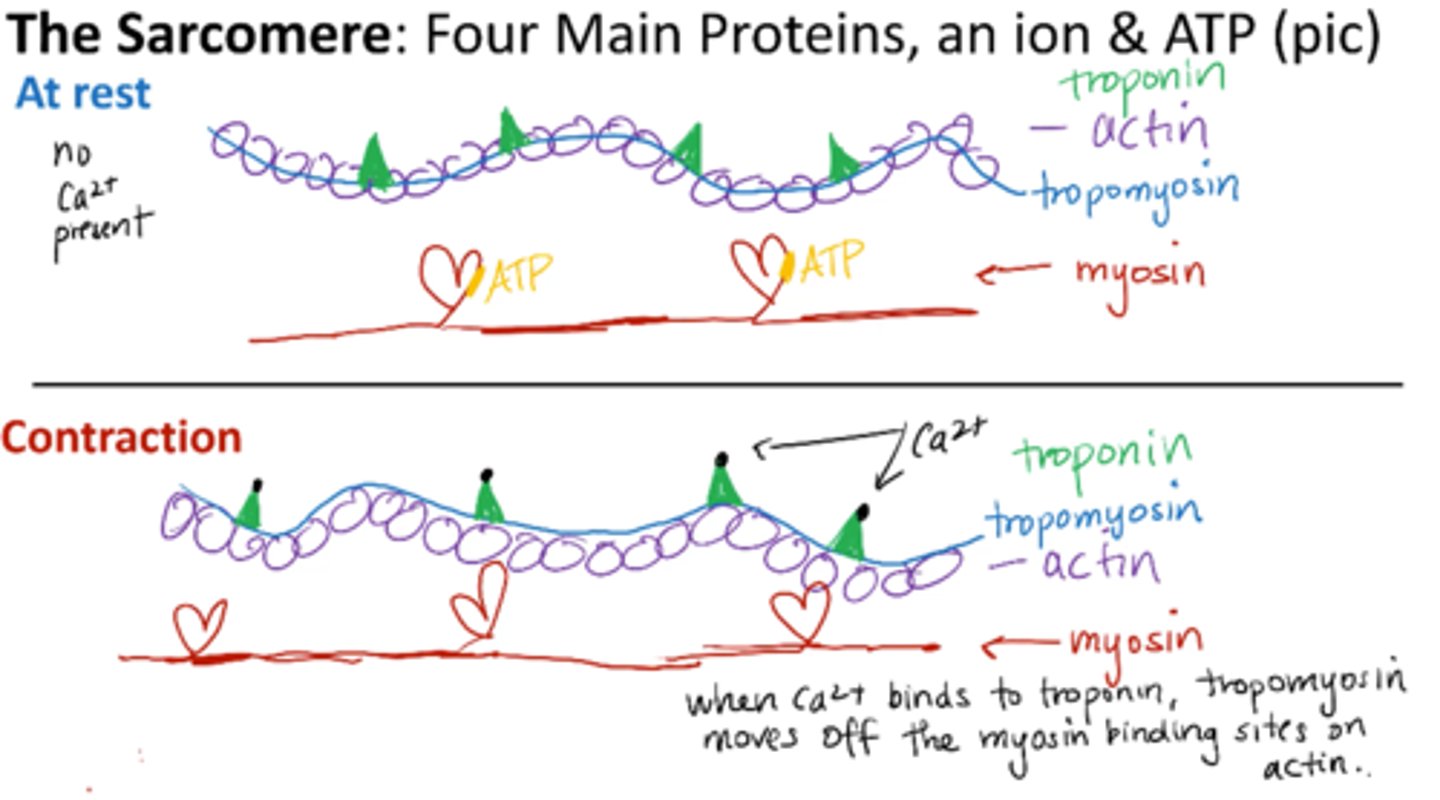
What is needed for muscle contraction?
Ca2+ - binds to troponin
ATP - binds to myosin
If calcium were unavailable, it would affect the structure/confirmation of which protein(s)?
A. troponin
B. tropomyosin
C. actin
D. myosin
E. all of them
all of them
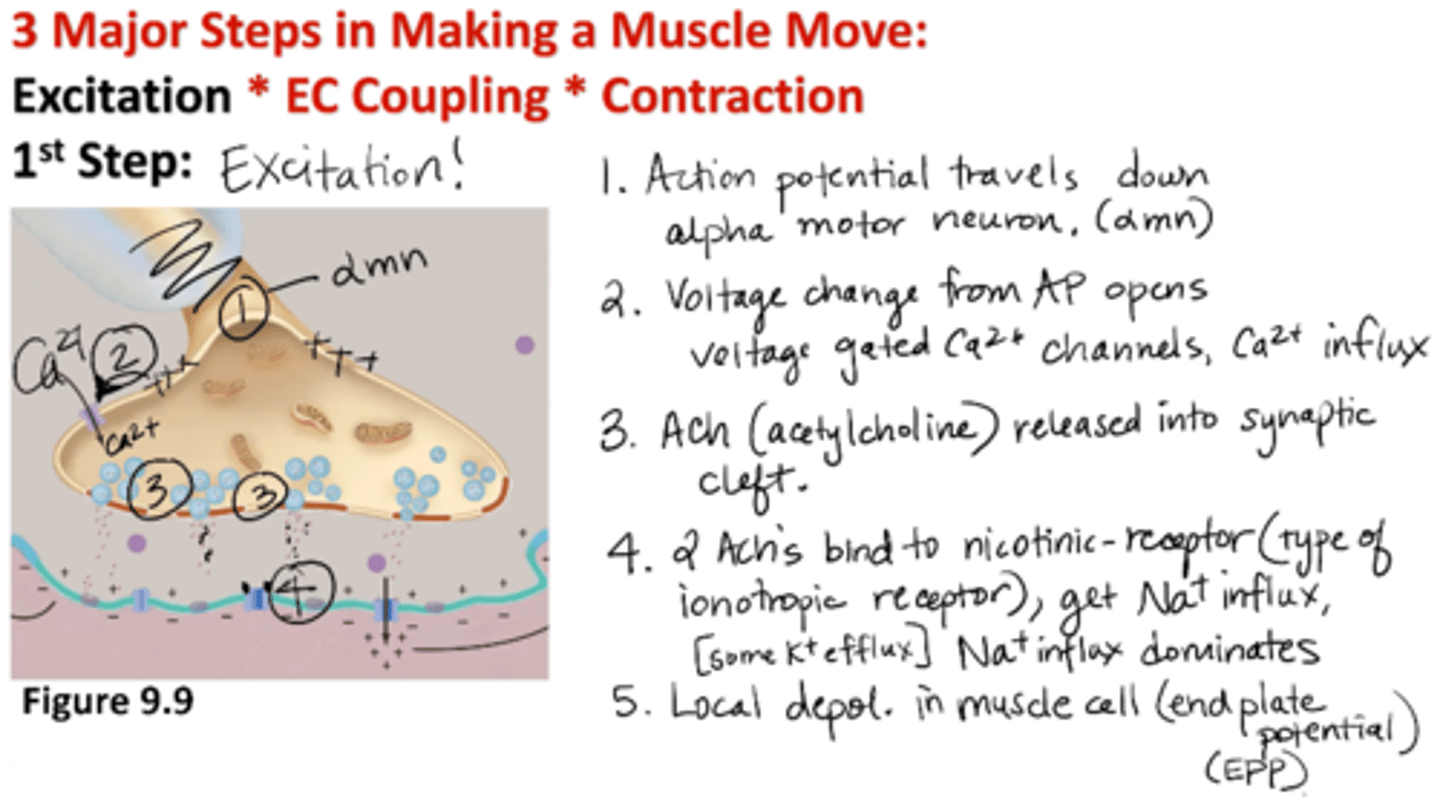
Excitation: 1st Step of Muscle Contraction
1) AP travels down alpha motor neuron
2) Voltage change from AP opens voltage-gated Ca2+ channels → Ca2+ influx
3) Acetylcholine (ACh) is release into synaptic cleft
4) 2 ACh’s bind to nicotinic receptor (type of ionotropic receptor) → Na+ influx (some K+ efflux); NA+ influx dominates
5) Local depolarization in muscle cell (end plate potential EPP)
A skeletal muscle deprived of adequate ATP supplies will...
A) immediately relax
B) release all actin-myosin bonds
C) enter a state where actin and myosin are unable to separate
D) fire many more action potentials than usual and enter a state of rigor
E) sequester all free calcium ions into the sarcoplasmic reticulum
C) enter a state where actin and myosin are unable to separate

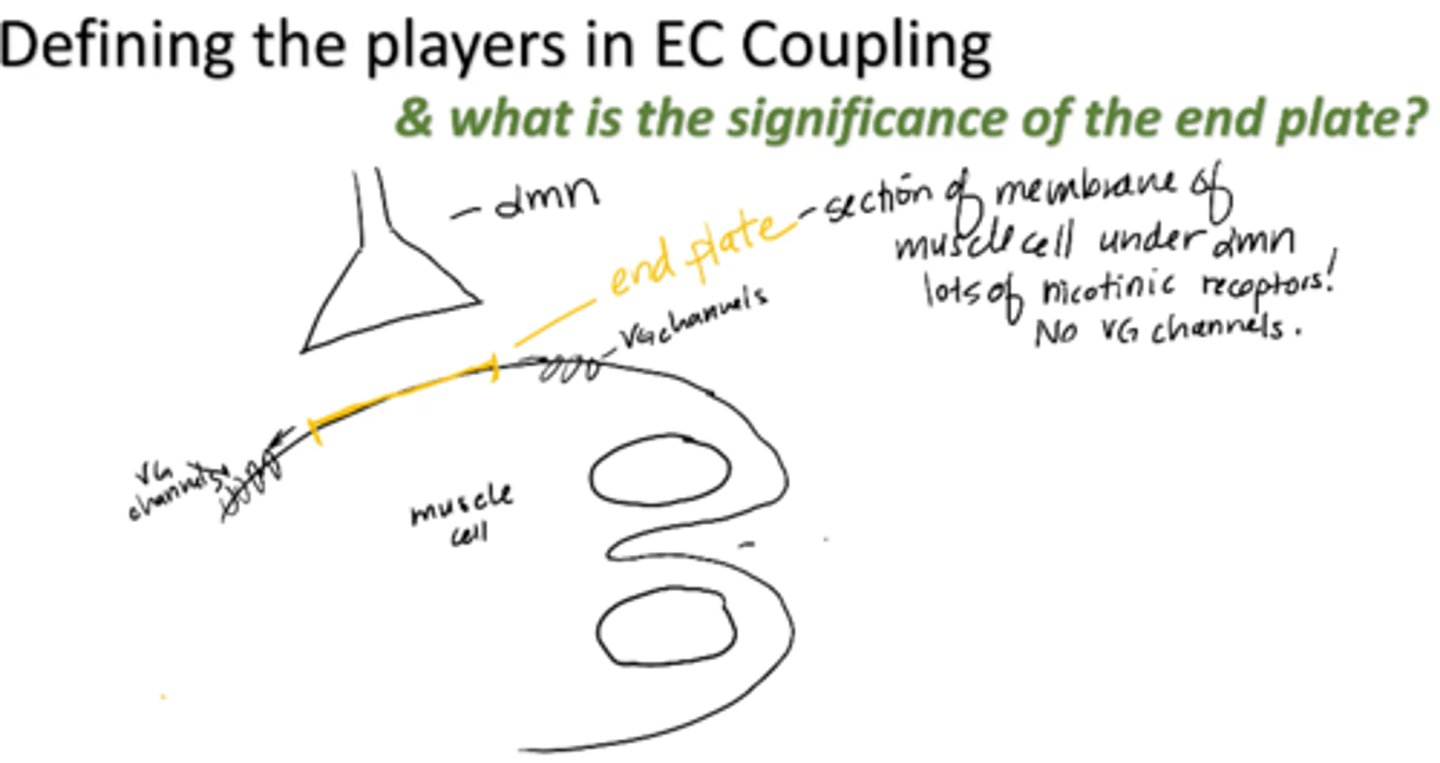
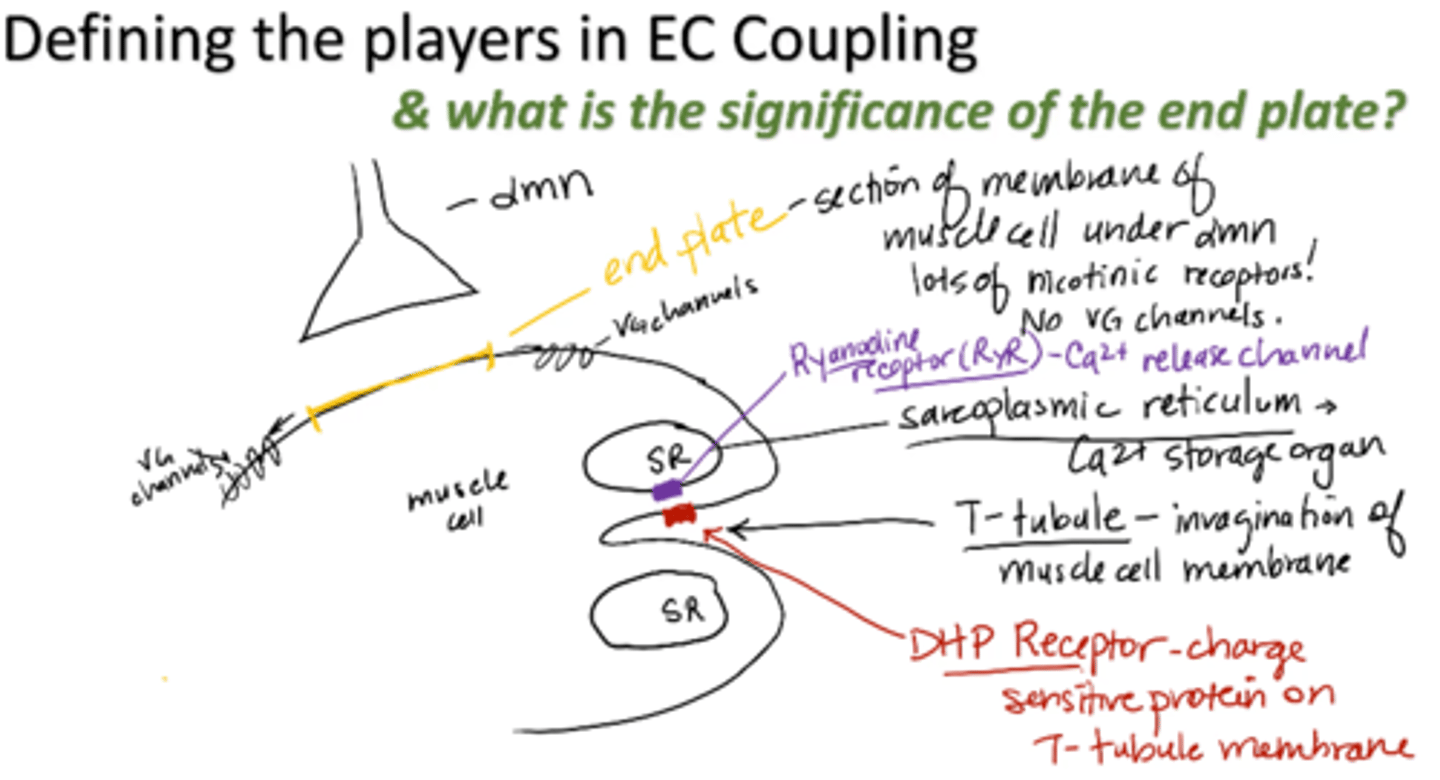
end plate
section of membrane of muscle cell under alpha motor neuron
- Lots of nicotinic receptors
- No VG channels
T-tubule
invagination of muscle cell membrane
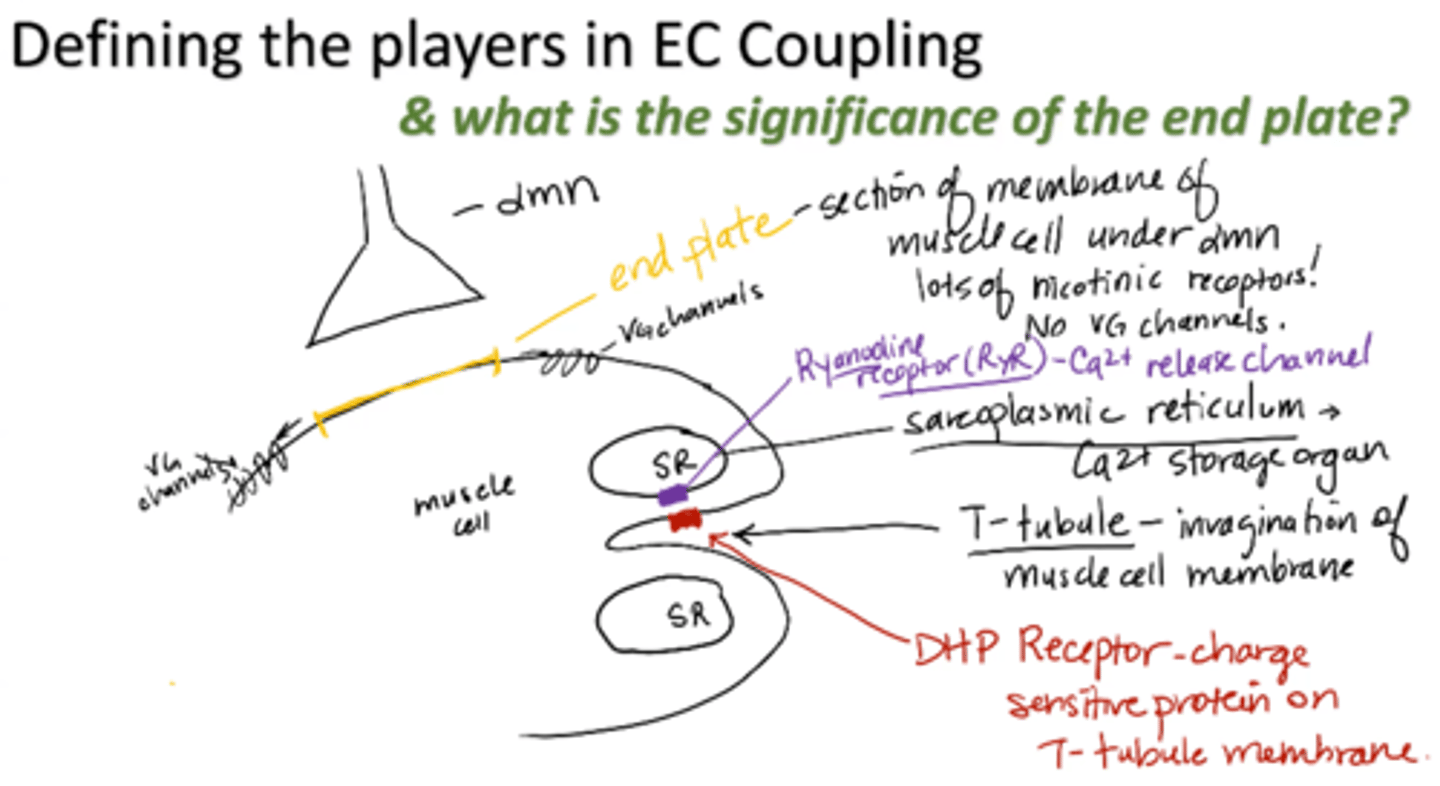
sarcoplasmic reticulum (SR)
calcium storage organ
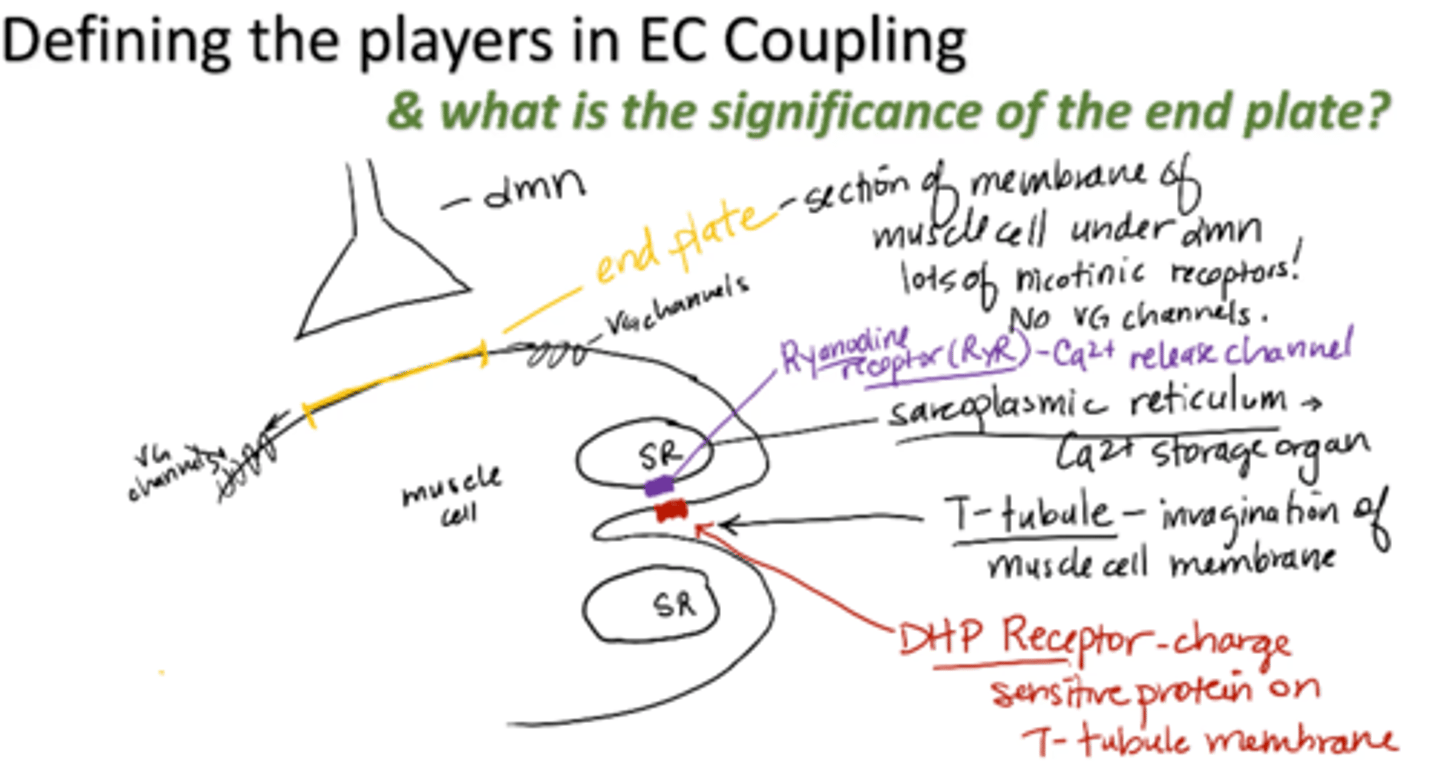
Ryanodine receptor (RyR)
calcium release channel
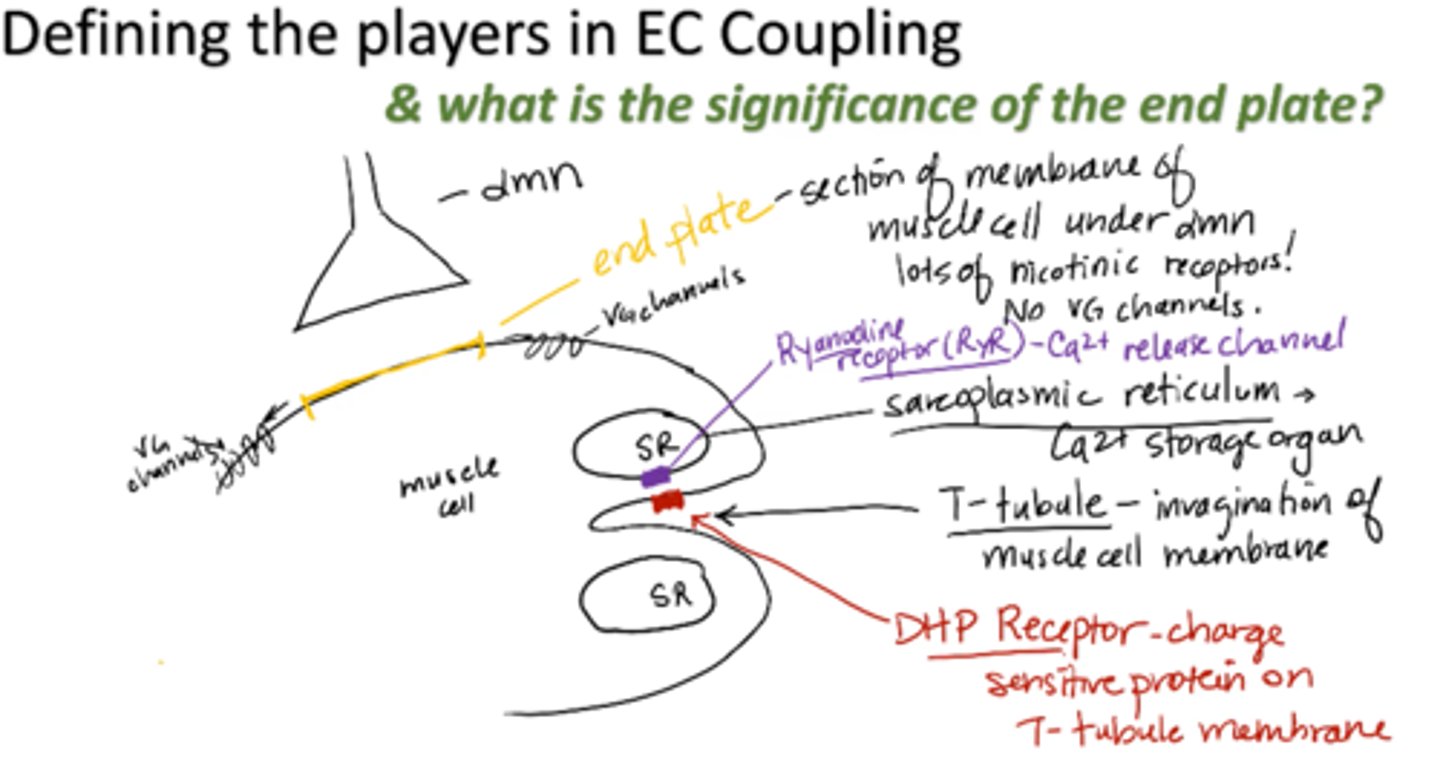
DHP receptor
charge sensitive protein on T-tubule membrane
EC Coupling: 2nd Step of Muscle Movement
1. AP propagates down muscle cell membrane into T-tubule (summation of EPPs)
2. Charge as a result of AP tells the DHP- receptor to communicate with Ryanodine receptor → tells RyR to open
3. Calcium rushes down its gradient, out of SR and into muscle cell cytoplasm
4. Calcium binds to troponin, preparing muscle for contraction
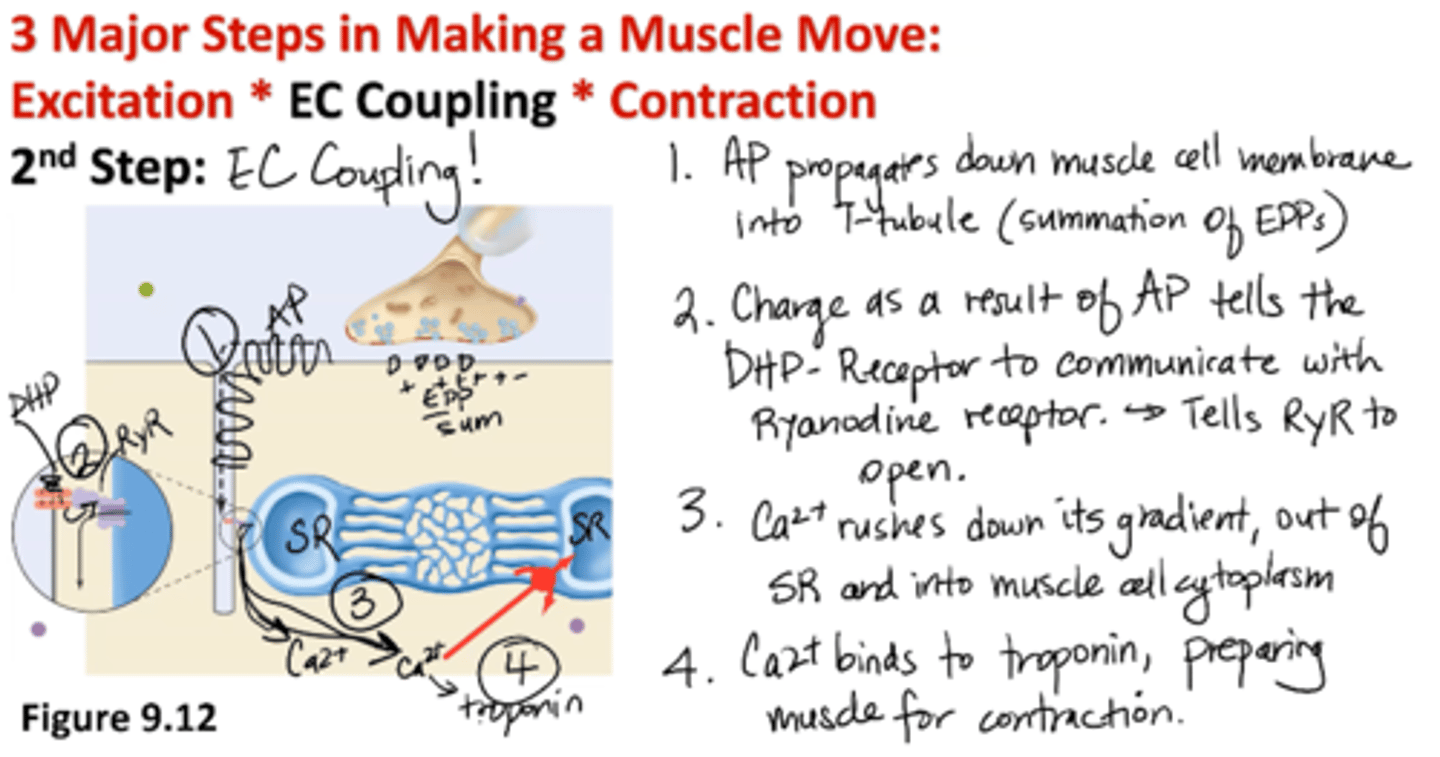
Where does the calcium for skeletal muscle CONTRACTION come from?
Ca2+ storage organelle, SR
Where does the calcium for skeletal muscle EXCITATION (nerve) come from?
extracellular environment
Contraction: 3rd Step of Muscle Movement
(Cross-Bridge Cycling) (Sliding Filament Theory)
1. Myosin, ADP, Pi (energized cross bridge) bind to actin
2. Actin, myosin, ADP, Pi → “crowded” force producing beginning → “Power stroke” release of ADP, Pi
3. Actin, myosin → main force producing step
+ ATP (new ATP binds to myosin)
4. Myosin, ATP → dissociated from actin (no more force)
Myosin ATPase hydrolyzes ATP → ADP + Pi (RATE LIMITING STEP)
Back to step 1: myosin, ADP, Pi (energized bridge)
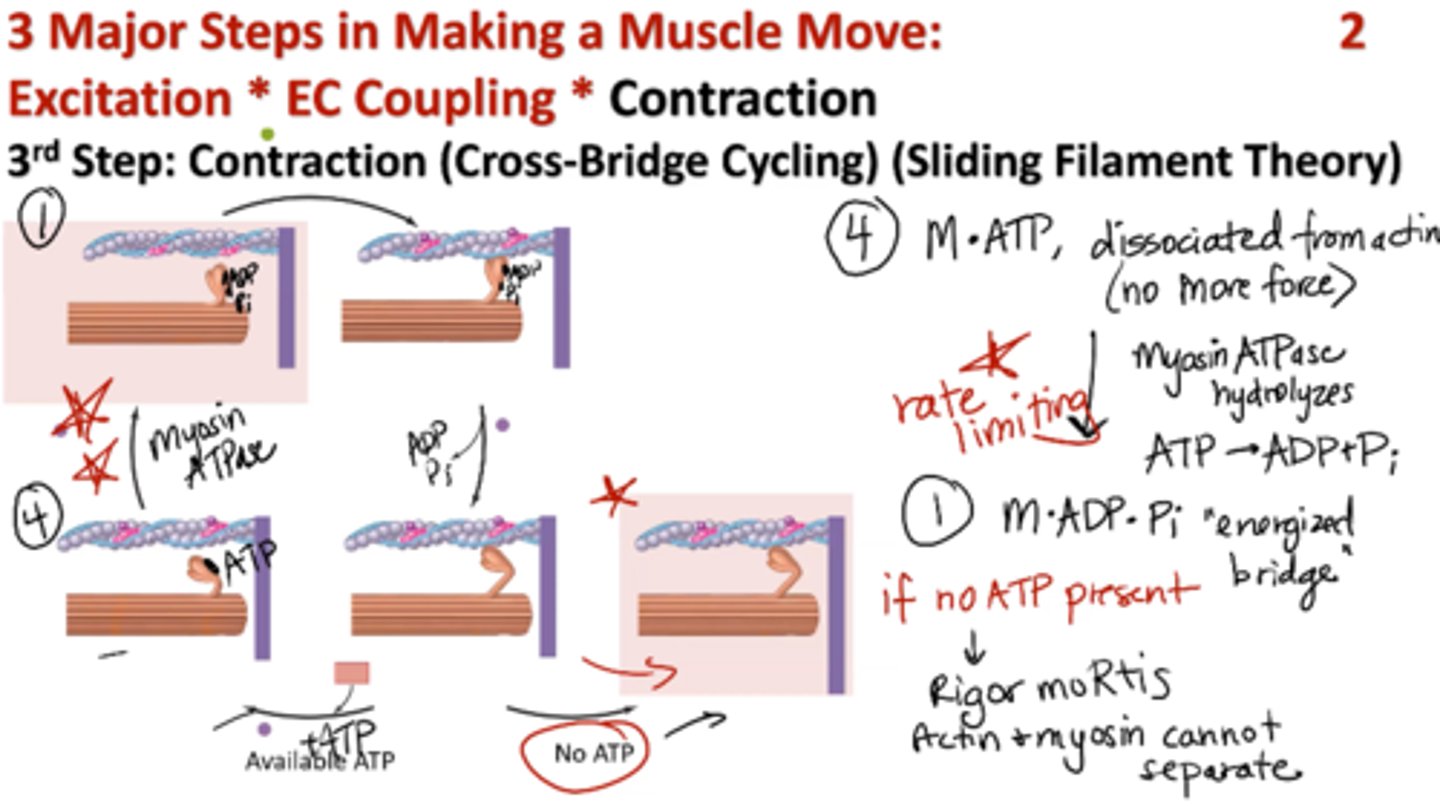
What happens if no ATP is present during contraction stage?
rigor mortis → actin and myosin cannot separate
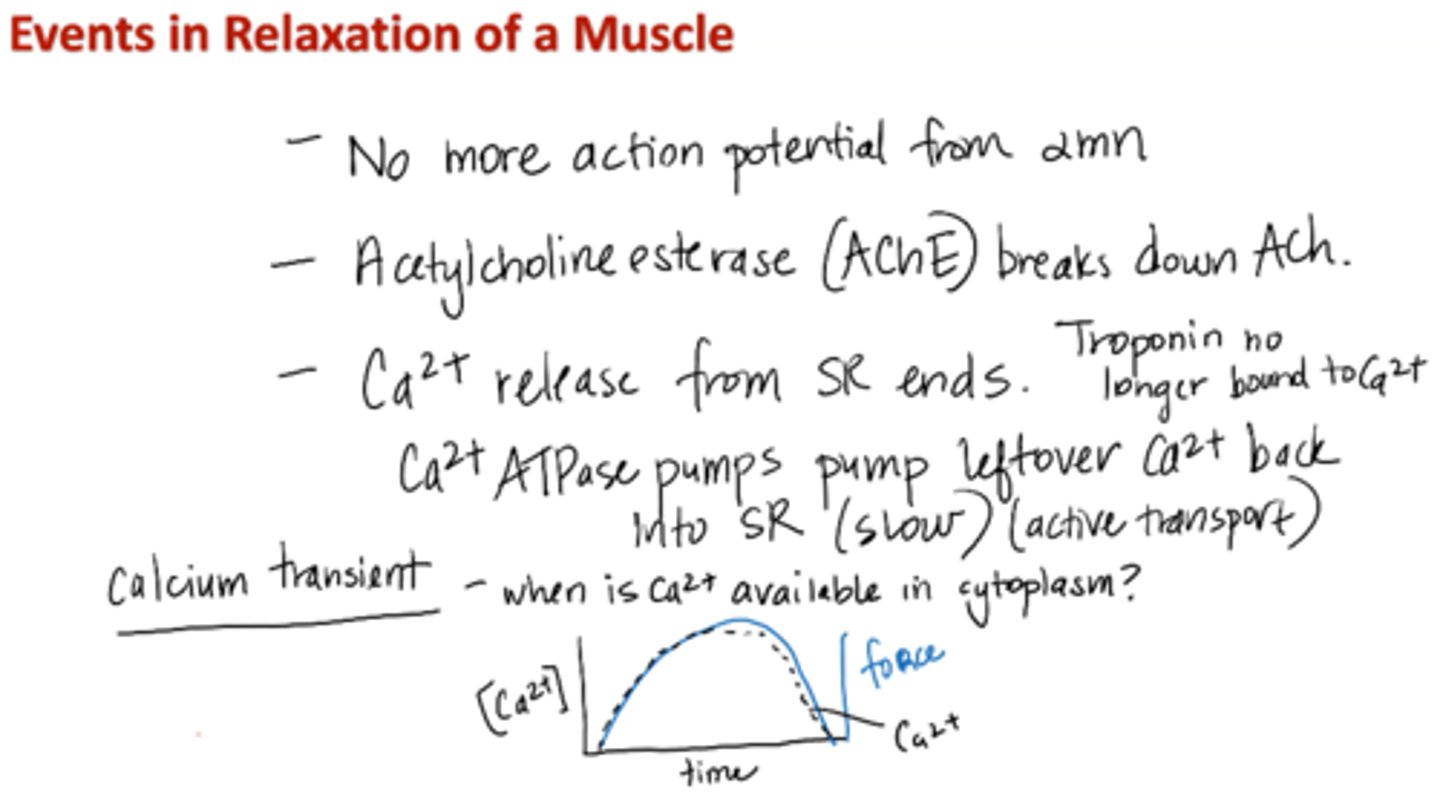
Events in relaxation of a muscle
1. No more action potential from alpha motor neuron
2. Acetylcholinesterase (AChE) breaks down ACh
3. Calcium release from SR ends
Calcium ATPase pumps pump leftover calcium back into SR (slow; active transport)
4. Troponin no longer bound to calcium
*Calcium transient – when is calcium available in cytoplasm?
Which of the following would cause constant contraction and now allow for relaxation?
A) poison that degrades ACh
B) something that renders AChE ineffective
C) Ca2+ ATPase pumps not being able to pump Ca2+ back into the SR
D) ATP remaining bound to myosin
E) B and C
E) B and C
B) something that renders AChE ineffective
C) Ca2+ ATPase pumps not being able to pump Ca2+ back into the SR

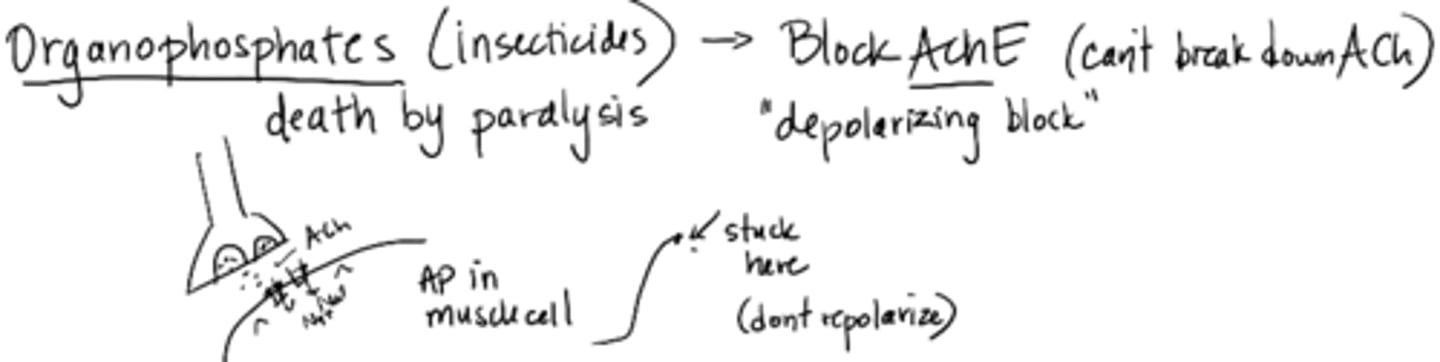
Organophosphates (insecticides)
death by paralysis
blocks AChE (cannot break down ACh)
depolarizing block; does not repolarize
Vecuronium
paralytic agent used to keep muscle quiet during surgery
ACh antagonist – blocks ACh binding to nicotinic-R (non-depolarizing block)

force / tension
# of actin-myosin bridges
velocity
rate of cross bridge cycling myosin ATPase enzyme
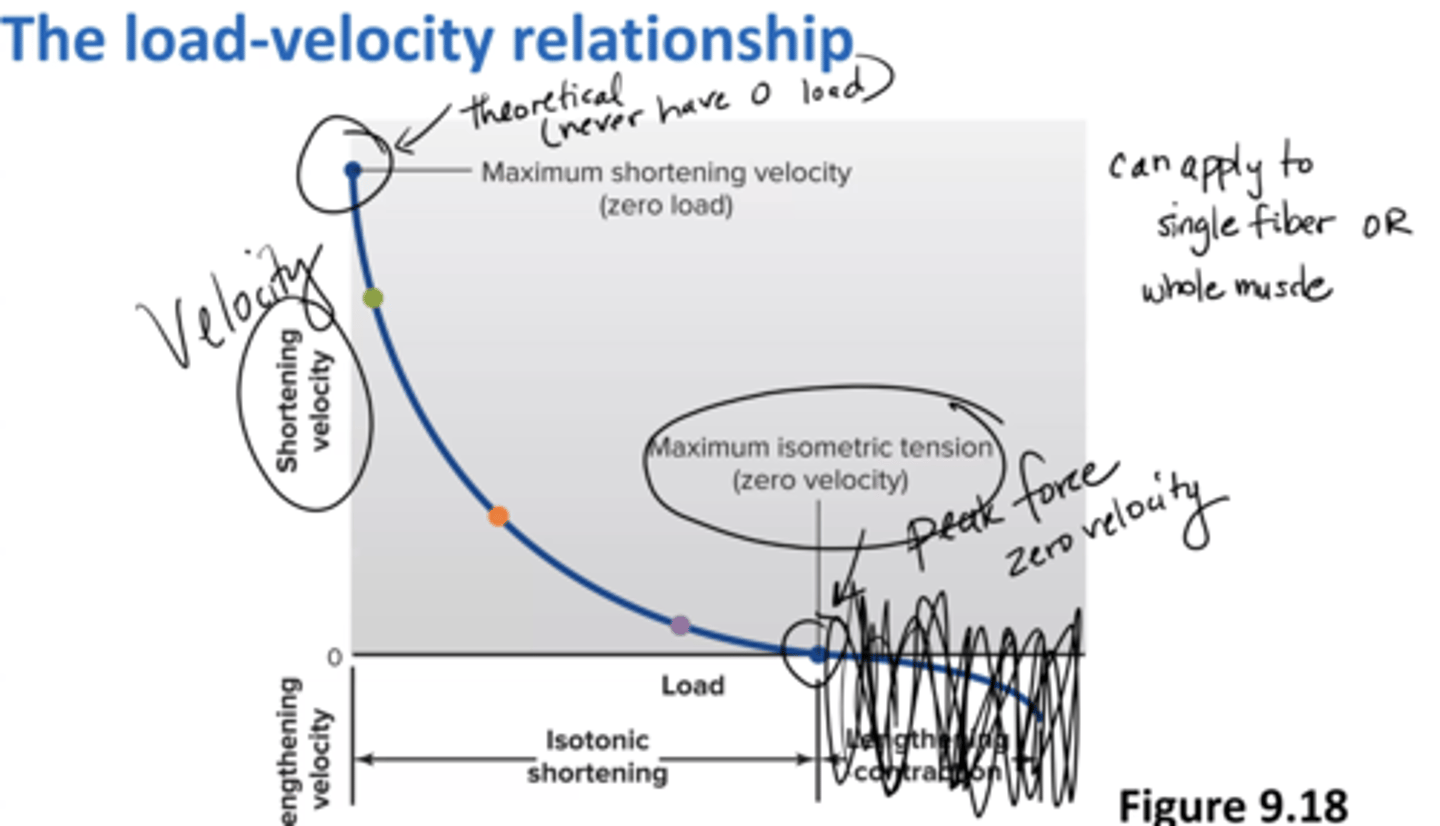
load-velocity relationship
Peak force = zero velocity (maximum isometric tension)
Peak velocity = zero load (maximum shortening velocity)
can apply to a single fiber or a whole muscle
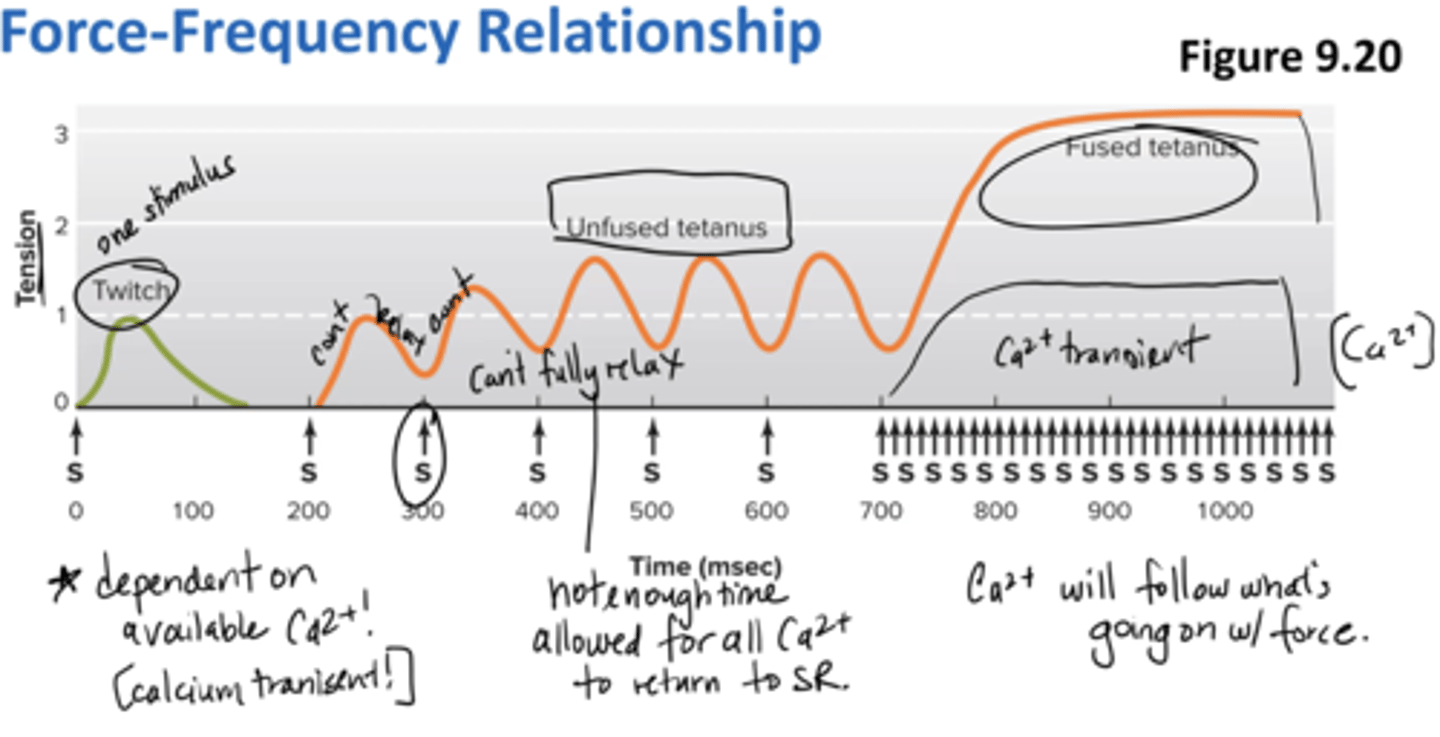
force-frequency relationship
dependent on available calcium (calcium transient)
unfused tetanus = not enough time allowed for all calcium to return to SR (can't fully relax)
fused tetanus = calcium will follow what's going on w/ force
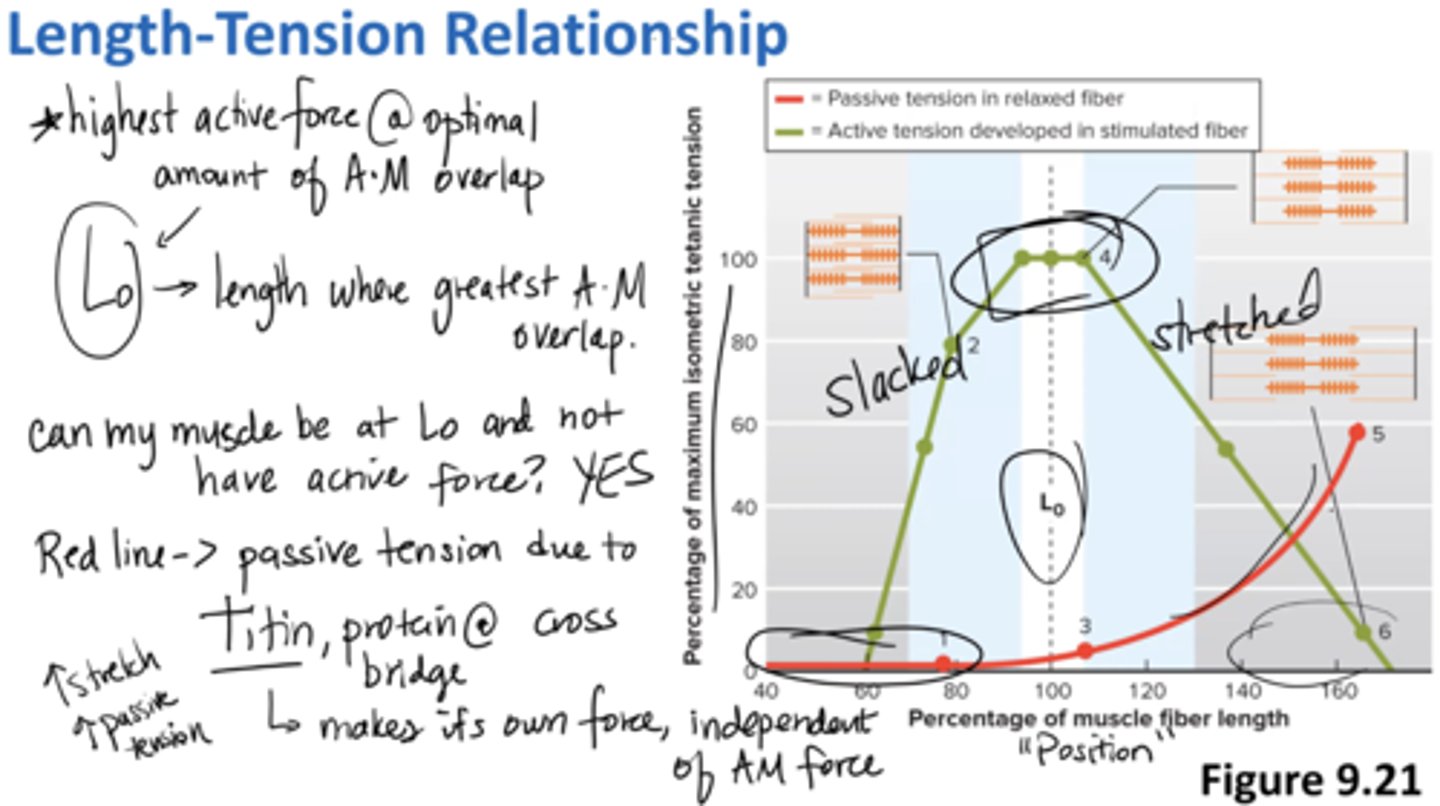
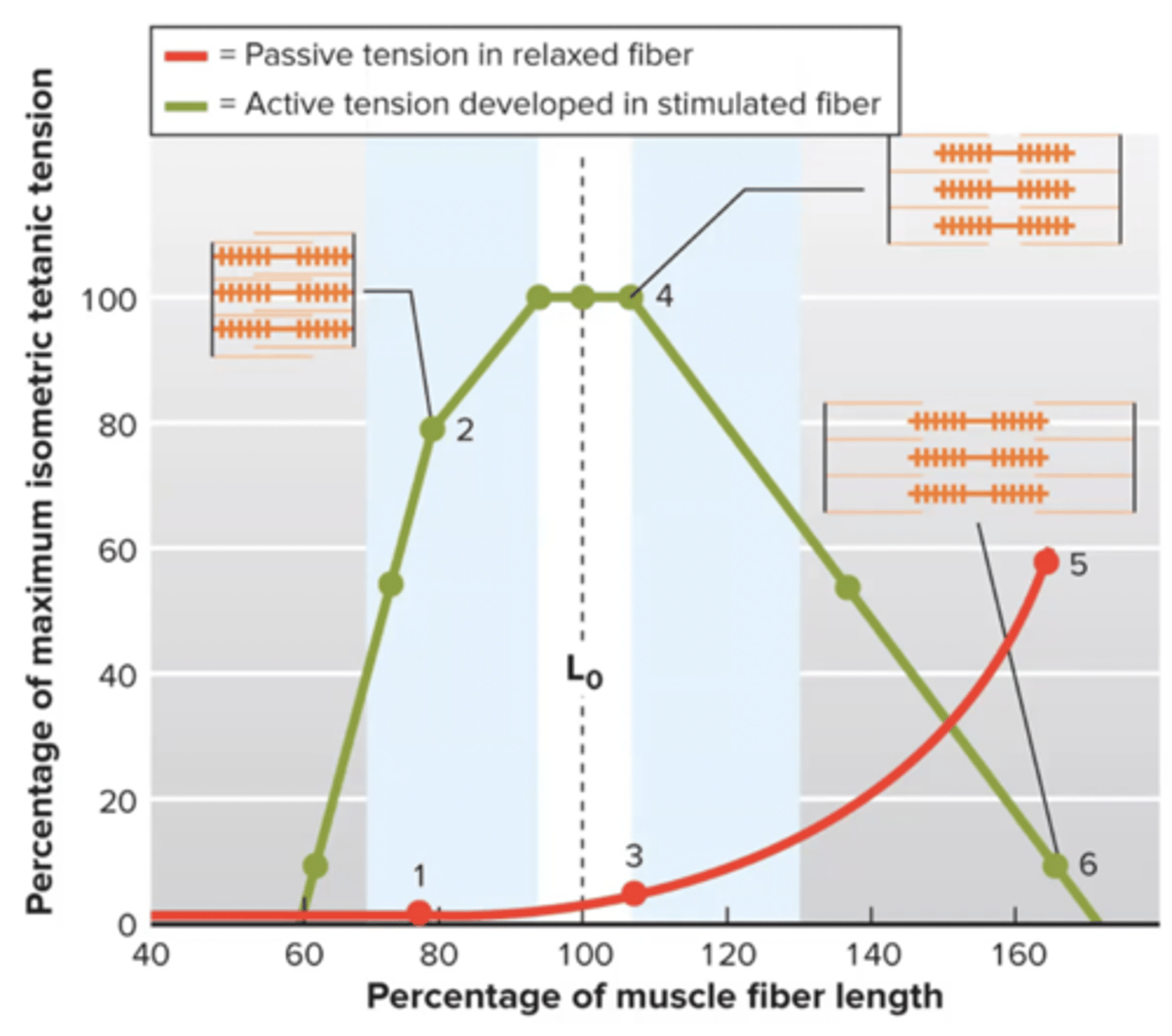
length-tension relationship
highest active force at optimal amount of actin, myosin overlap
Lo = length where greatest actin + myosin overlap
can my muscle be at Lo and not have active force? YES
red line → passive tension due to Titin (protein at cross bridge)
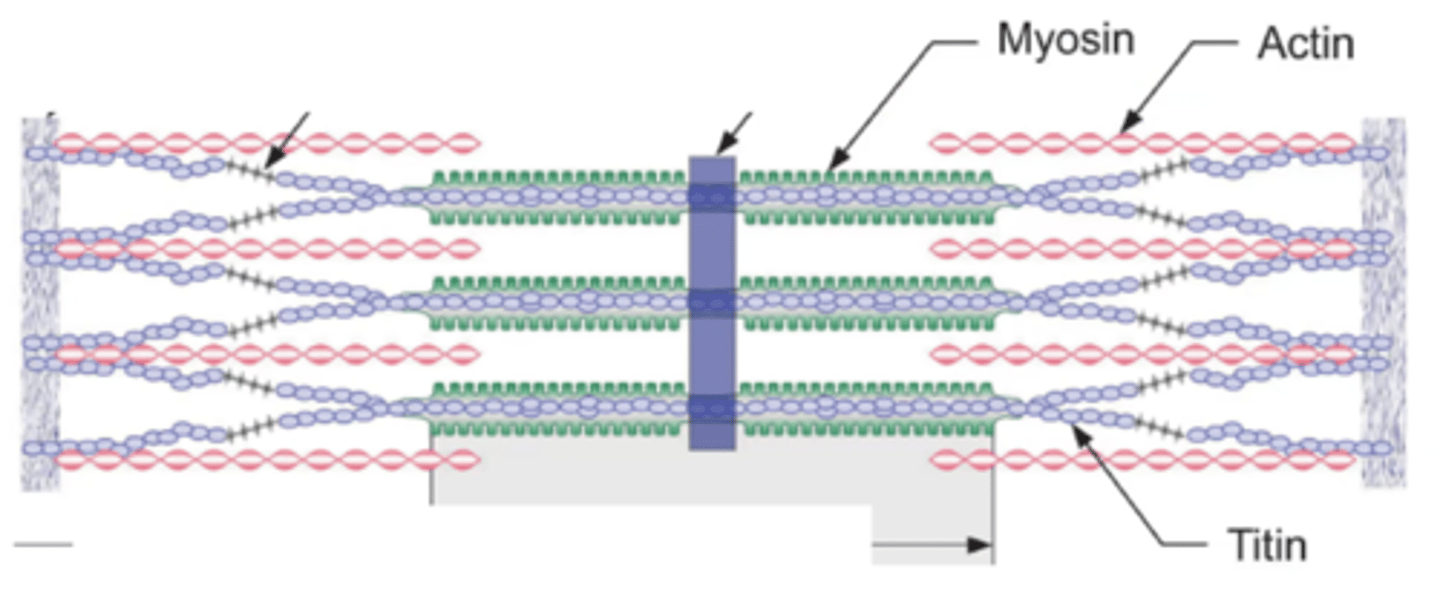
Titin
protein at cross bridge that makes it own force, independent of actin-myosin force
located at the sarcomere
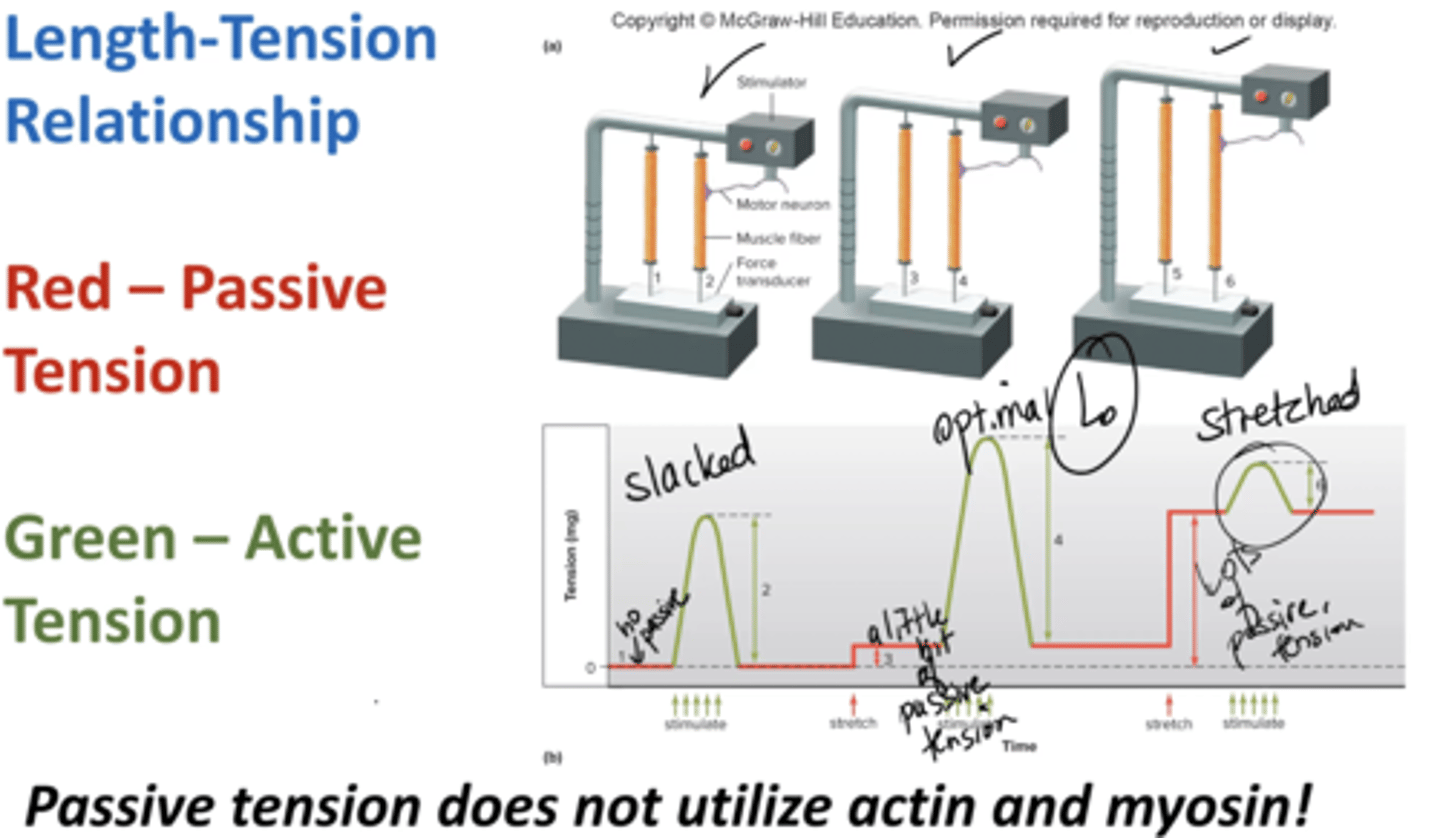
passive tension
tension applied to load when a muscle is stretched but not stimulated
does not utilize actin and myosin
governed by Titin
ie stretch
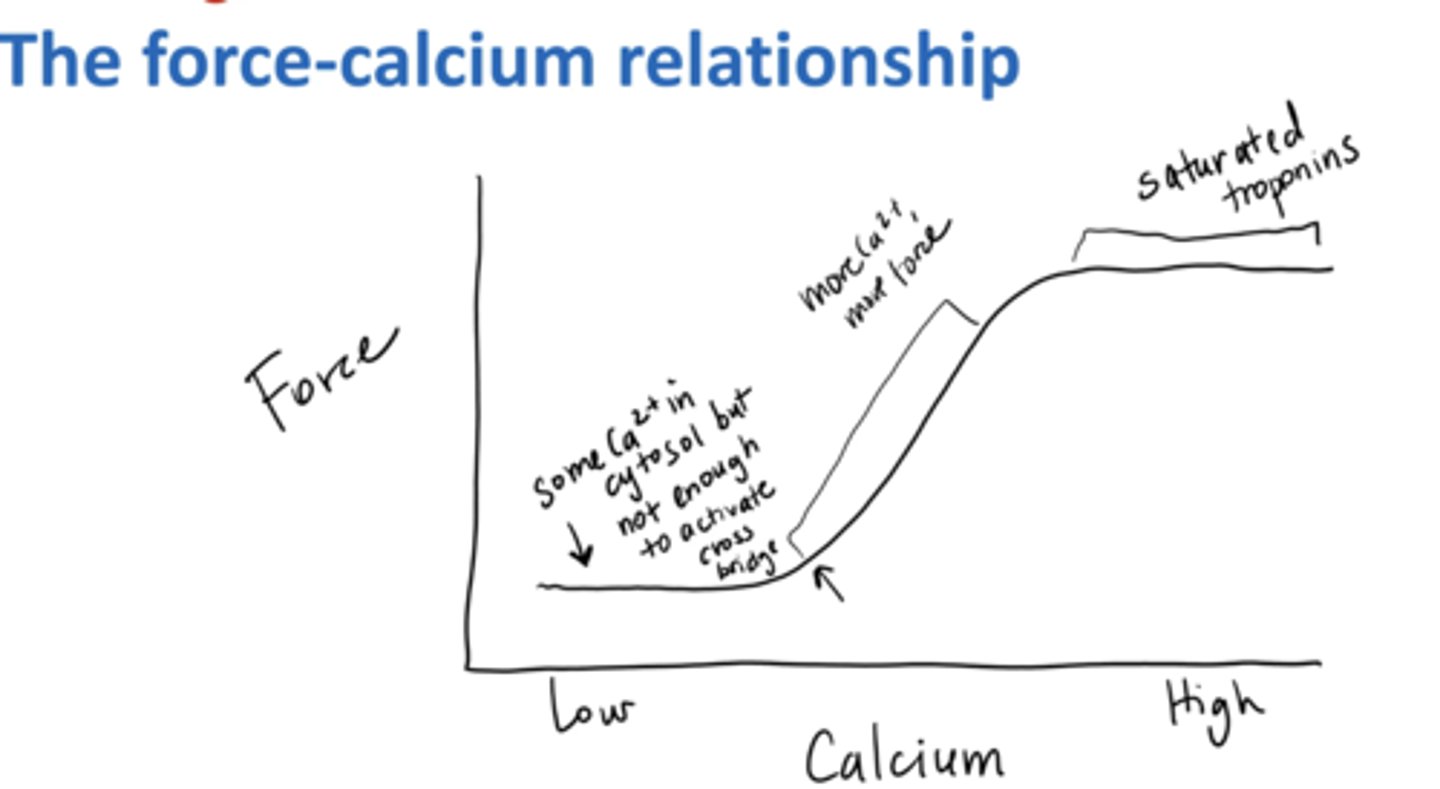
More Ca2+ can
sometimes/sometimes not produce more Force
More Ca2+ requires more Force
@ peak force Troponin is saturated so more Ca2+ has no affect
force-calcium relationship
The higher the calcium → the more the force
two exceptions:
1) not enough for thin filament regulatory proteins to move
2) troponin saturated w/ Ca2+
A cat is laying down sleeping and its gastrocnemius (calf) muscle fibers are right around Lo. What type of force production is happening?
A) none, the cat is sleeping
B) a small amount of passive tension
C) peak force since the fibers are at Lo
B) a small amount of passive tension
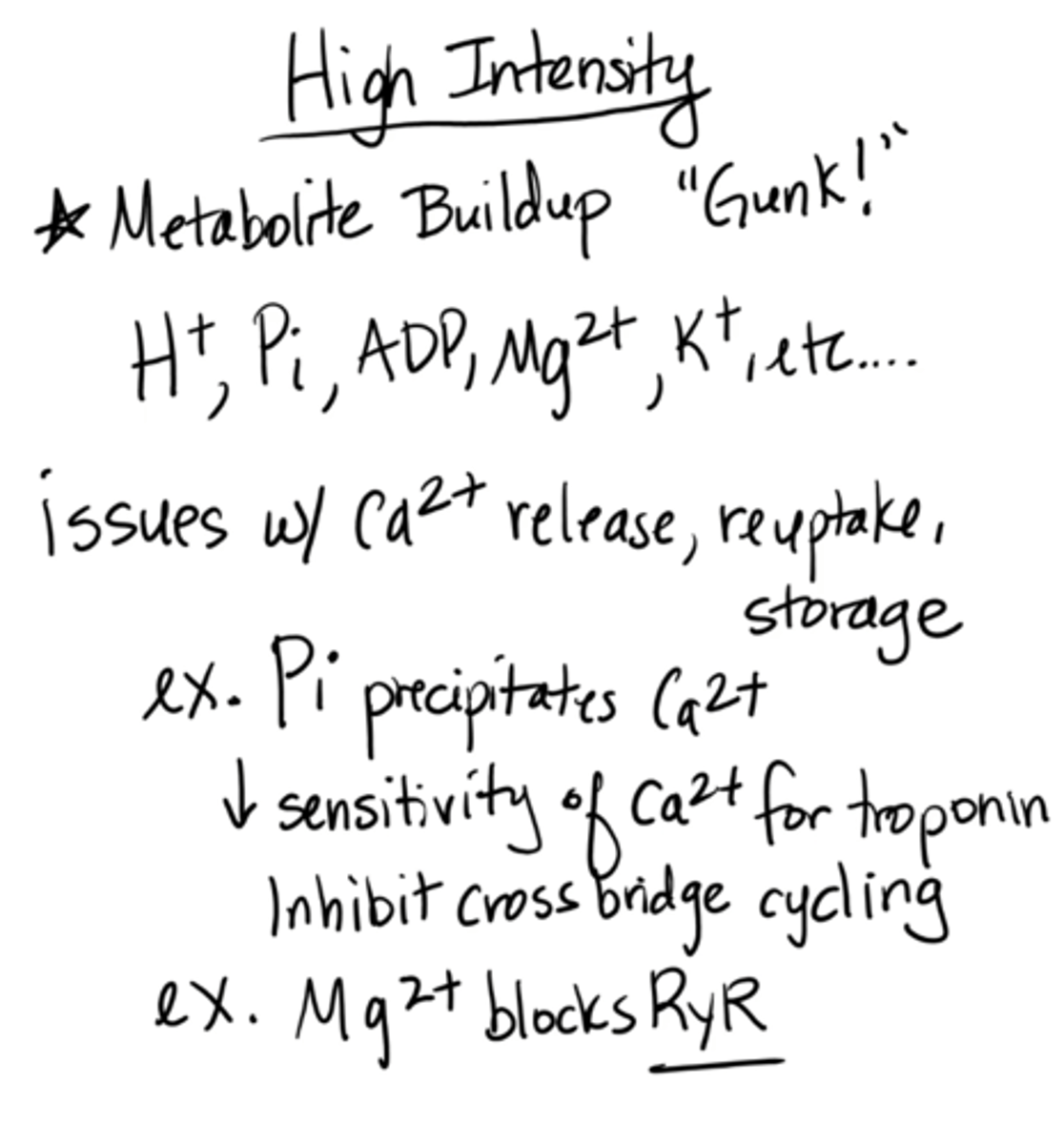
high intensity muscle fatigue
Metabolite buildup "gunk!"
(H+, Pi, ADP, Mg2+, K+)
decrease in Ca2+ release, reuptake, storage
ex: decrease Ca2+ sensitivity to troponin (inhibits cross-bridge cycling)
ex: Mg2+ blocks RyR
e.g. benching
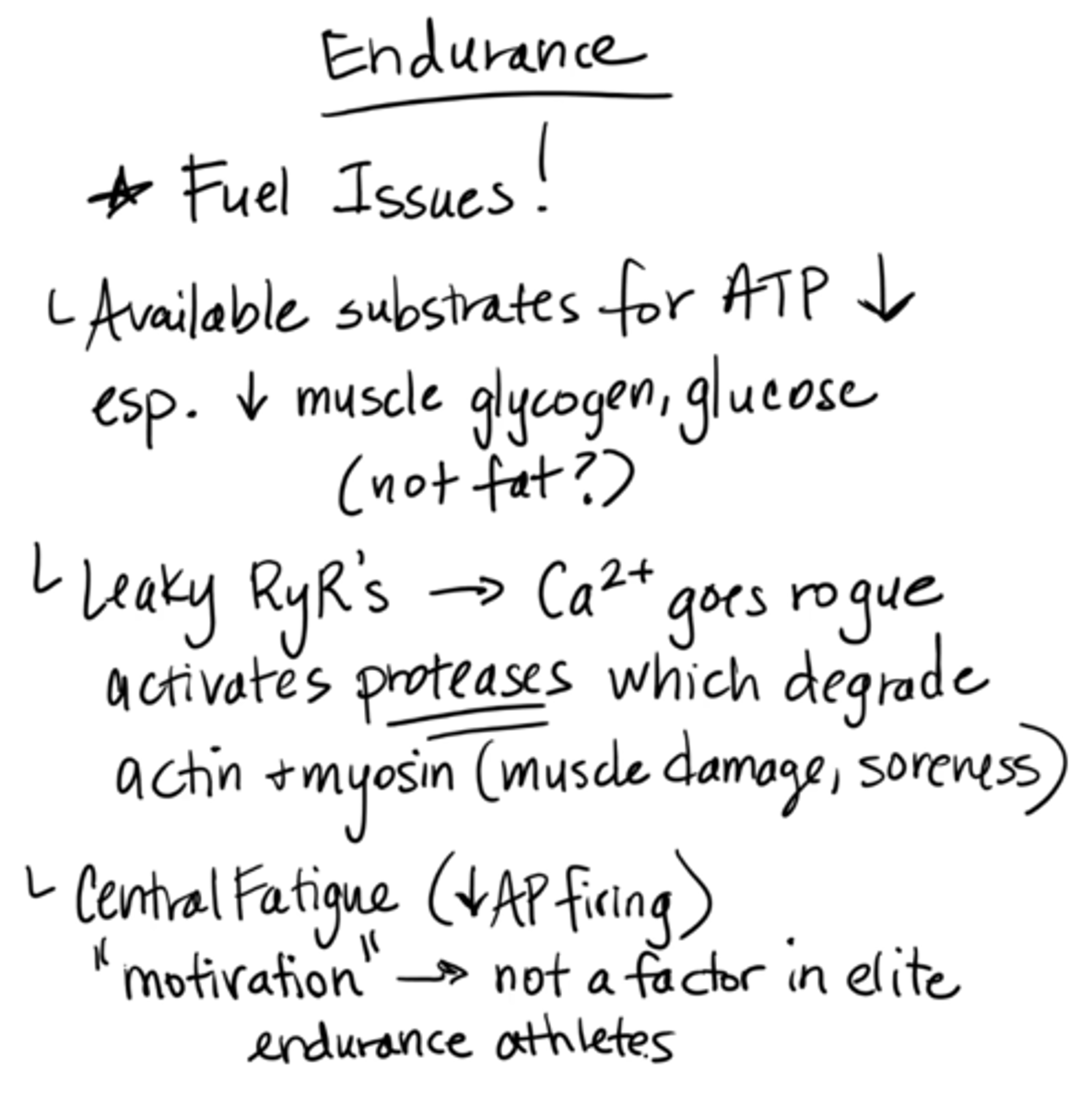
endurance fatigue
fuel issues
available substrates for ATP decrease, esp. decrease muscle glycogen, glucose (not fat)
leaky RyRs → calcium goes rogue, activates proteases which degrade actin + myosin (muscle damage, soreness)
central fatigue (decreased AP firing)
"motivation" → not a factor in elite endurance athletes
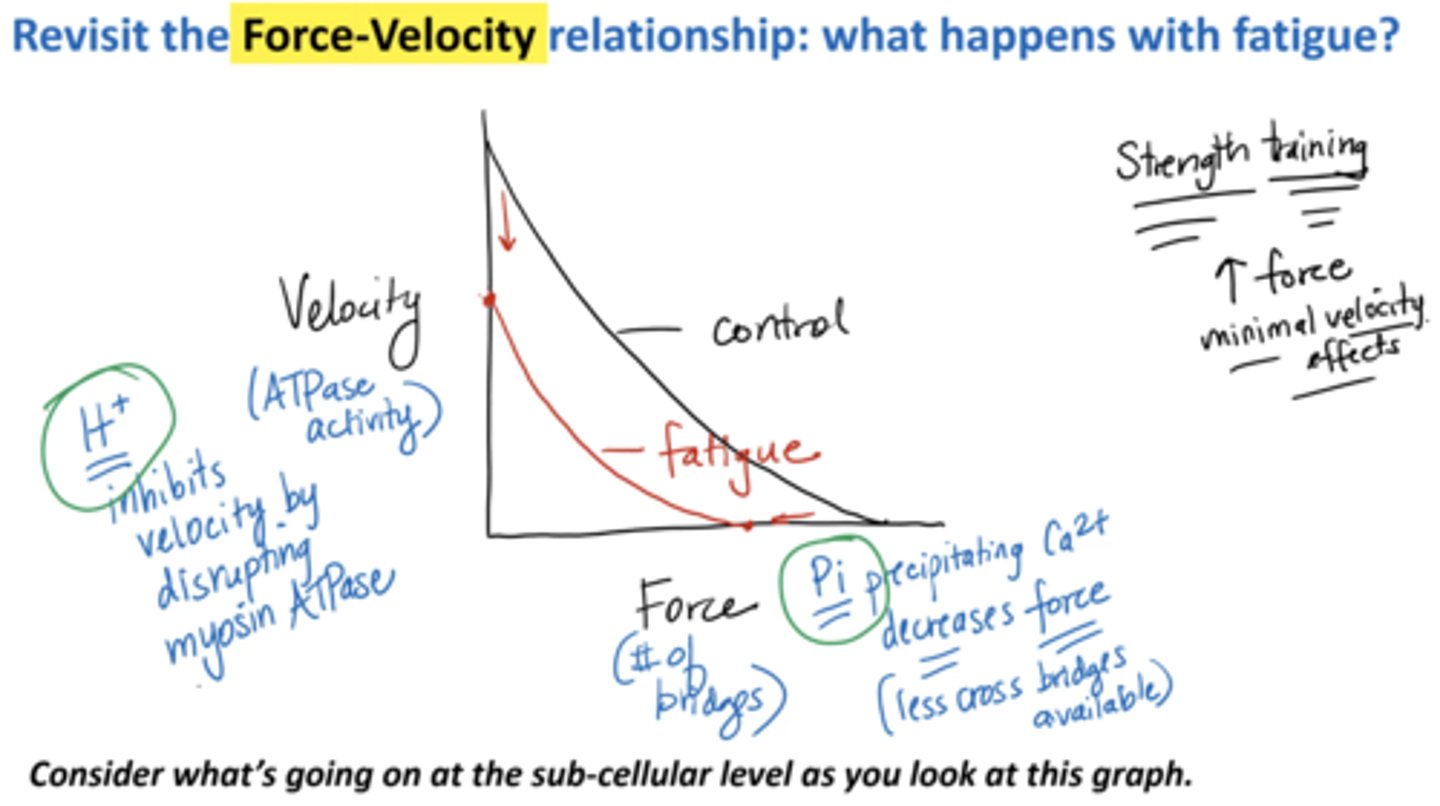
Revisit the force-velocity relationship: what happens with fatigue?
Velocity:
H+ inhibits velocity by disrupting myosin ATPase
Force (# of bridges):
Pi precipitating calcium decreases force (less cross bridges available)
strength training increases minimal velocity effects