Isomers
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What is an isomer?
Isomers are molecules with the same molecular formula but a different arragement of atoms within the molecule.
What are the 2 types of isomers?
Structural isomers
Stereoisomers
What are structural isomers?
Structural isomers have the same molecular formula but different structural formulas i.e. the way the atoms are arranged.
What are the 3 types of structural isomers?
Chain isomerism
Positional isomerism
Functional group isomerism
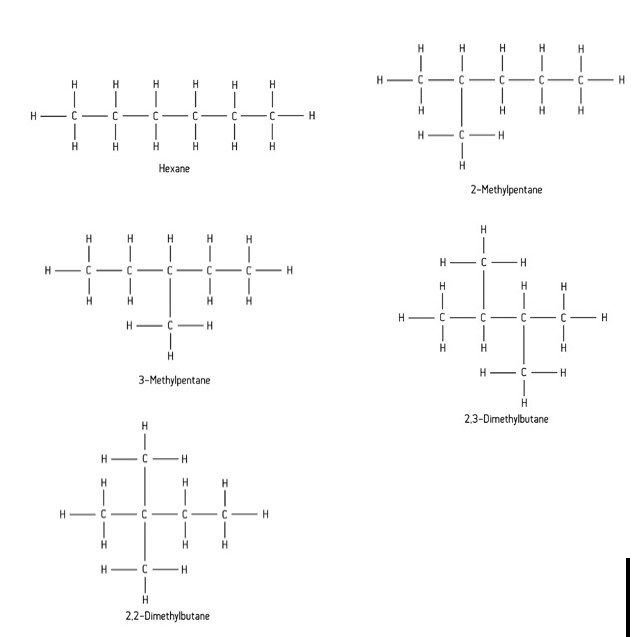
What is chain isomerism?
This occurs when the carbon chain within the molecules is arranged differently. This is usually because one isomer has branches instead of a straight chain.
Examples of isomers of hexane:
What is positional isomerism?
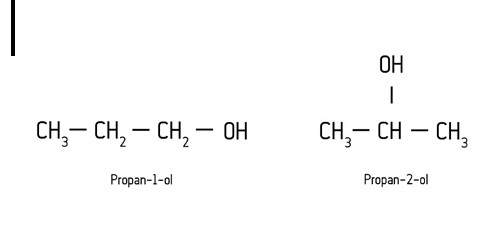
This occurs when the functional group is attached to the main chain at different locations.
Examples of isomers of propanol:
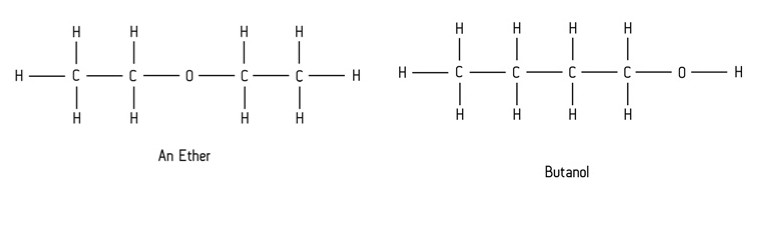
What is functional group isomerism?
This occurs when compounds have the same molecular formula, but different functional groups (usually within those containing oxygen).
Explain the difference in the boiling points of isomers of an organic compound.
“Straight” chain isomers have higher boiling points than branched chain isomers.
The greater the degree of branching, the lower the boiling point.
Branching decreases the effectiveness of the attractive intermolecular forces.
Less energy is required to seperate the molecules.
Are there any differences in the chemical properties of isomers of an organic compound?
Most isomers show similar chemical properties if the same functional group is present.
Give one example of stereoisomerism.
E/Z isomerism.
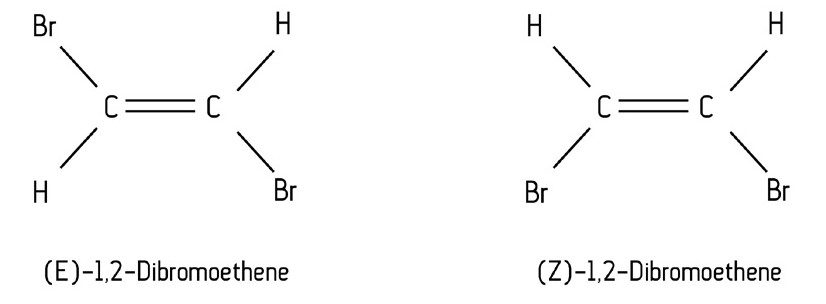
What is E/Z isomerism?
This type of isomerism occurs in alkenes because there is restricted rotation of C=C double bonds.
In alkanes, the single bond allows for free rotation of carbon atoms. However, when you have a double bond, the atoms are unable to rotate, meaning they are in fixed positions. This gives rise to isomers as the atoms exist in different places in space.
Example: 1,2-dibromoethene:
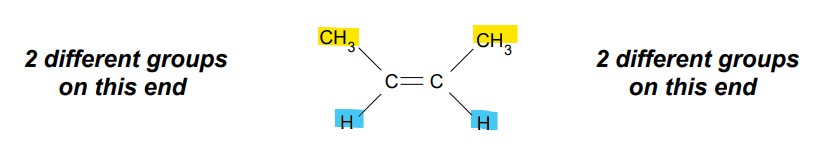
What conditions must be met for E/Z isomerism to occur?
There must be 2 different groups / atoms on each carbon in the double bond.
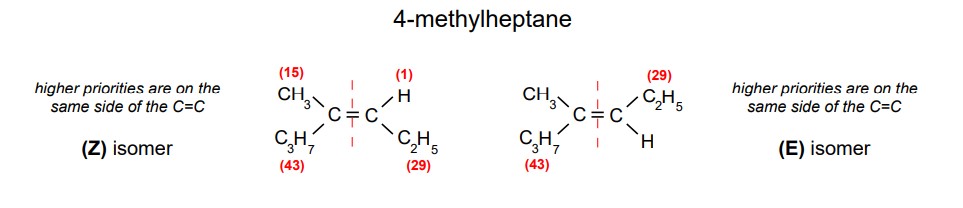
How do you determine which atoms/groups have highest priority when naming E/Z isomers?
Determine which groups / atoms have the highest atomic number.
Highest mass= Highest priority
Lower mass= Lower priority
How do you know if you have a Z isomer?
The higher priority groups are on the same side of the C=C double bond.
How do you know if you have a E isomer?
The higher priority groups are on opposite sides of the C=C double bond.
Describe the properties of E/Z isomers.
The different orientation of the functional groups can mean that E-Z isomers can have different physical and chemical properties.
Physical properties of the different isomers will also be different, e.g. boiling point. E- isomers tend to pack together better, so they will have stronger intermolecular forces and higher melting and boiling temperatures.