CHM2210 Functional Groups
1/43
Earn XP
Description and Tags
Organic Chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
44 Terms
hydrocarbons
only contain hydrogen and carbon
heteroatom
an atom that is not hydrogen or carbon
amide
Nitrogen atom bonded to a carbonyl (C=O) group, and a hydrogen and/or R group
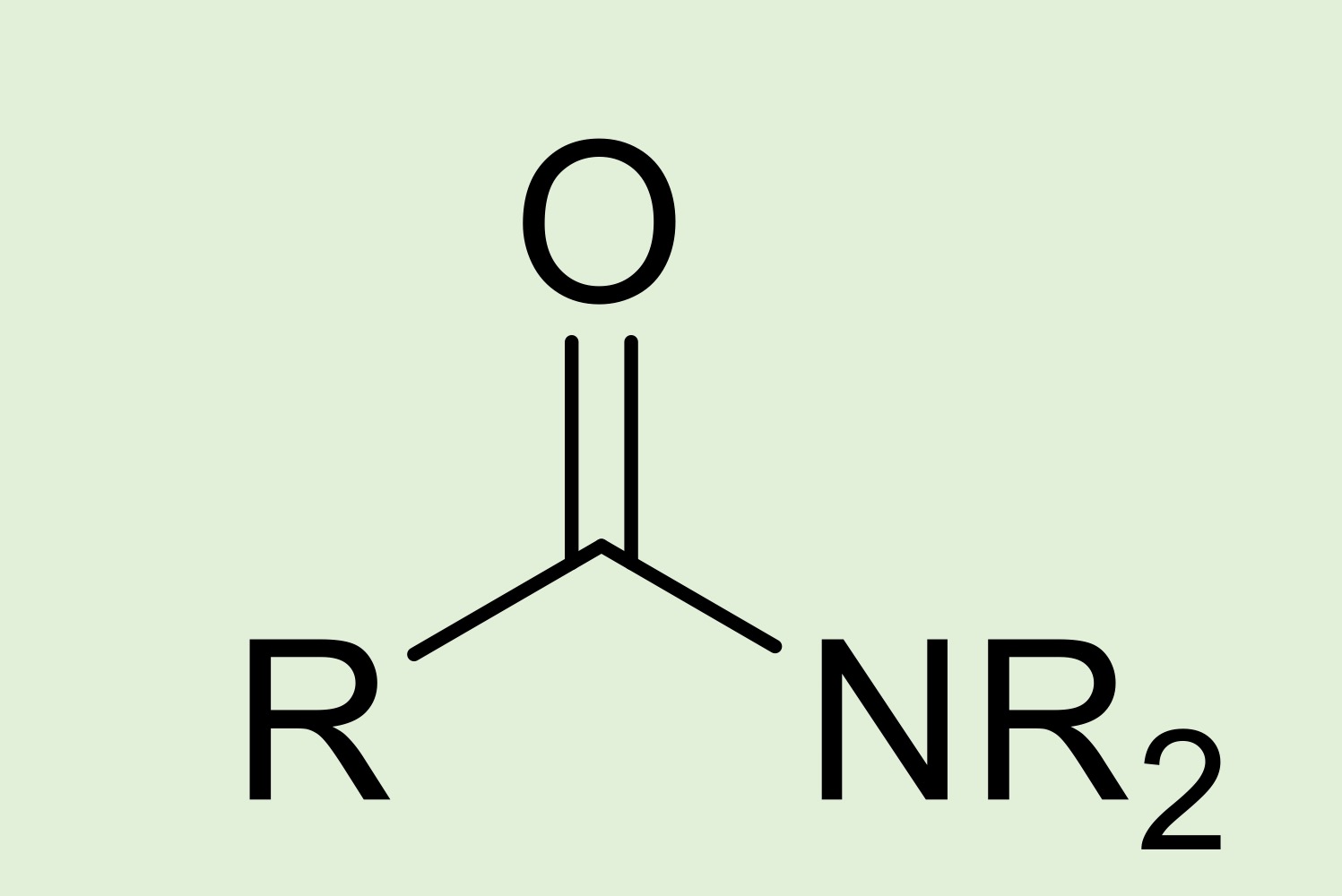
amide
alkane
hydrocarbon where carbons are single bonded
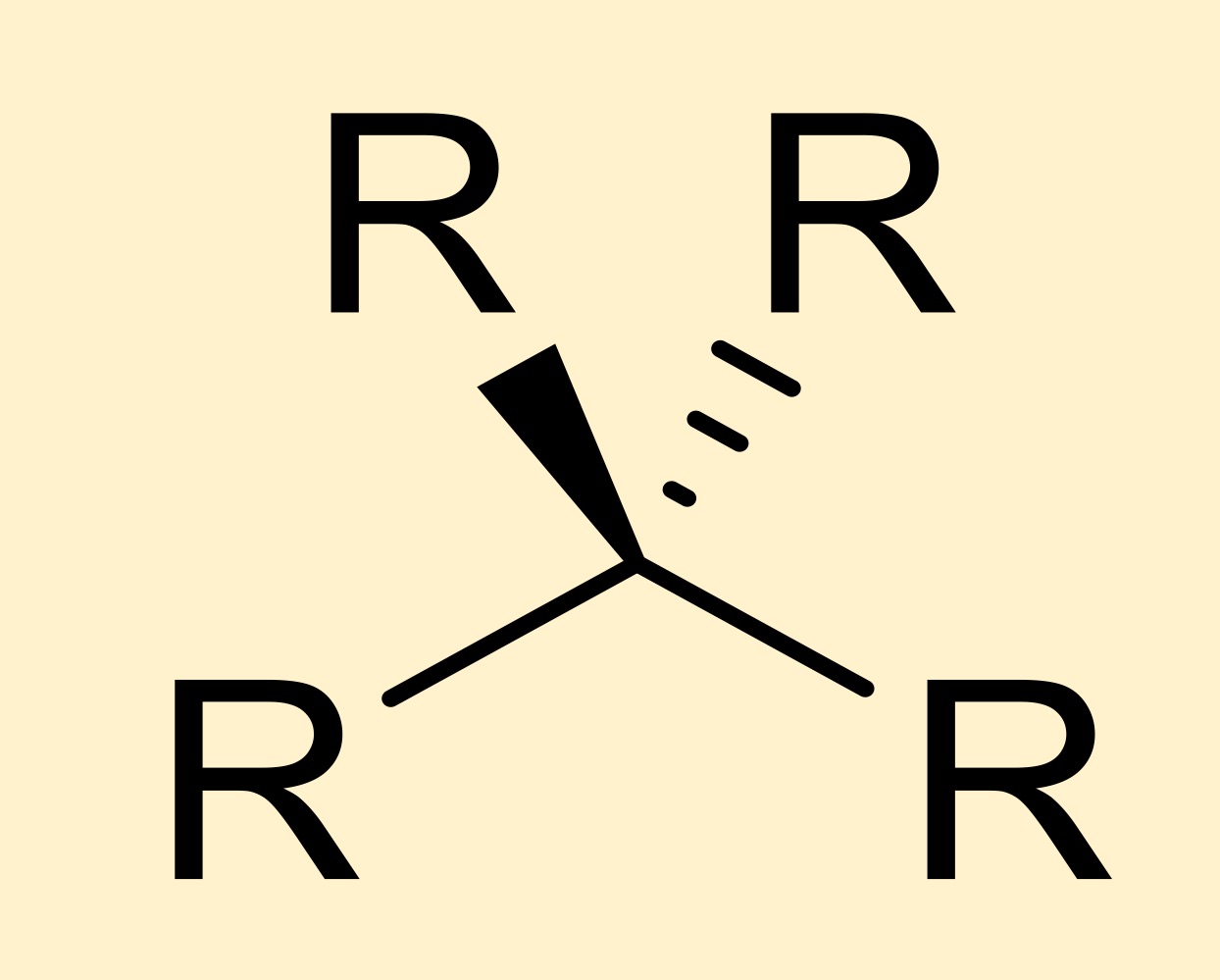
alkane
alkene
hydrocarbon where carbons are double bonded
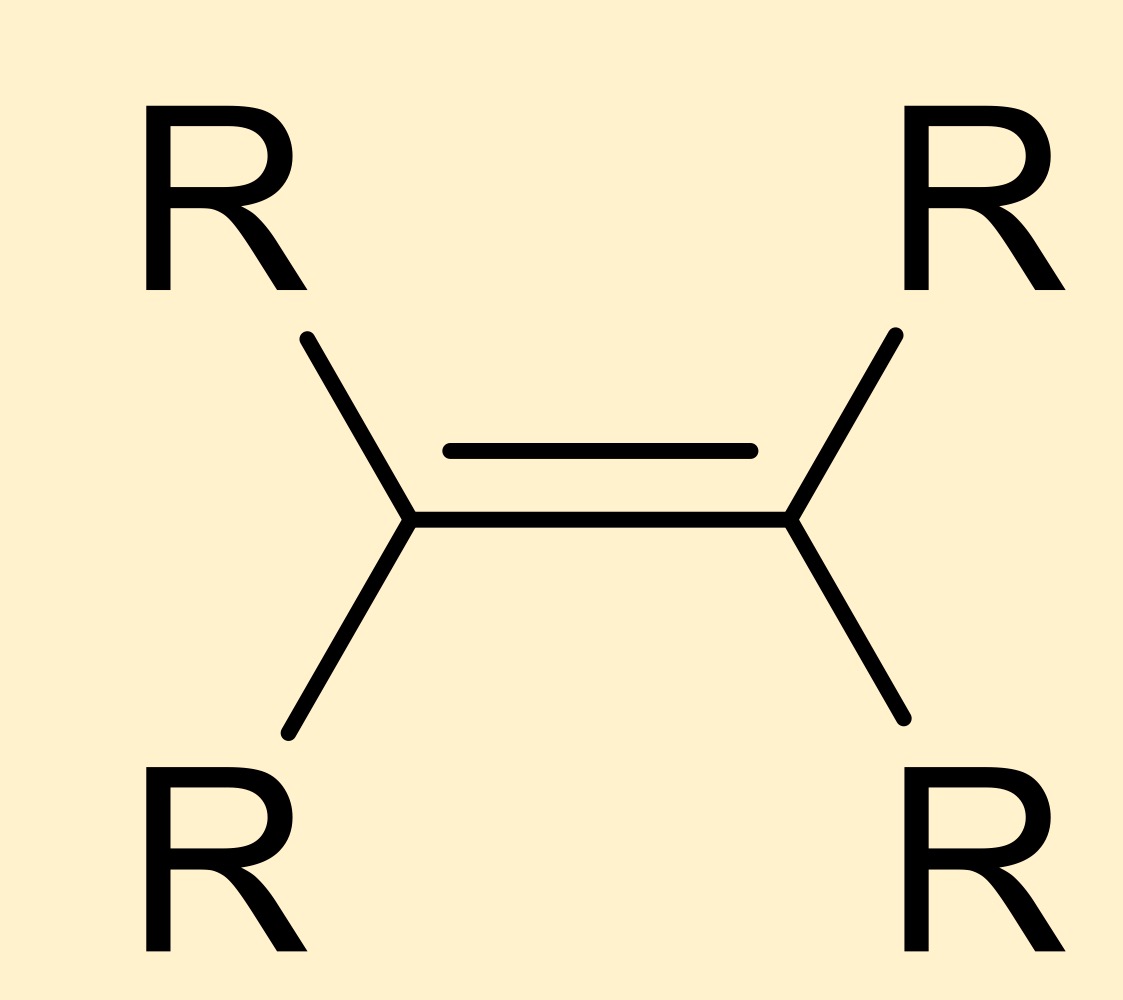
alkene
alkyne
hydrocarbon where carbons are triple bonded
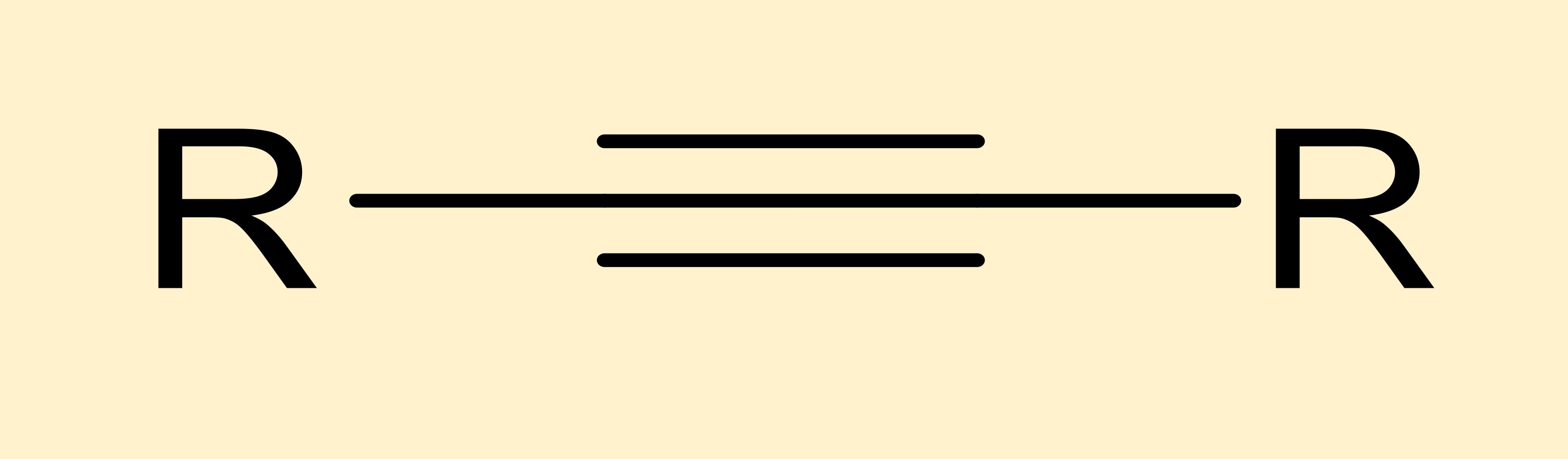
alkyne
arene (benzene)
hydrocarbon containing a stable, pi bonded ring of carbons
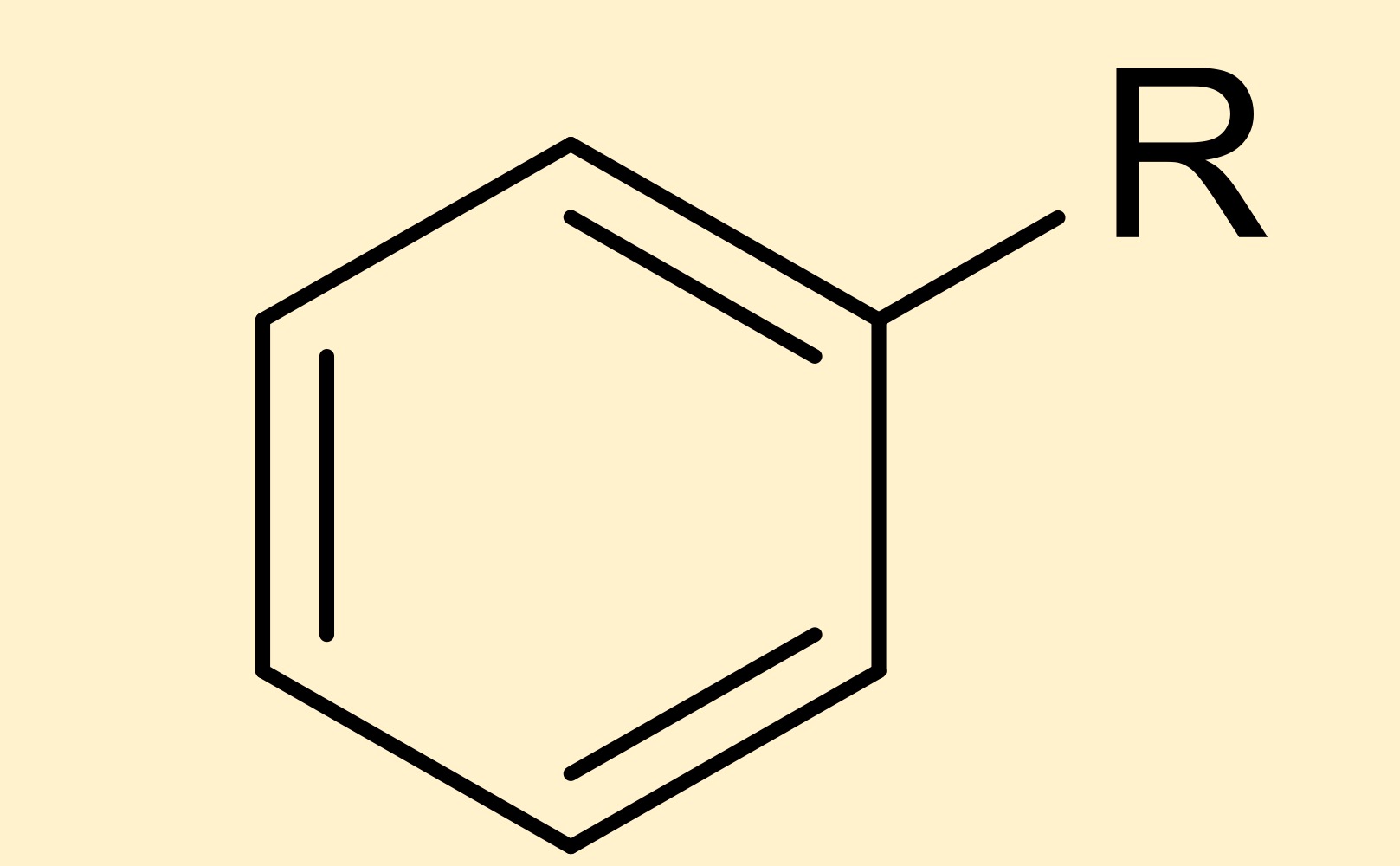
arene
R
H, alkyl
X
F, Cl, Br, I
alcohol
hydroxyl attached to a carbon atom
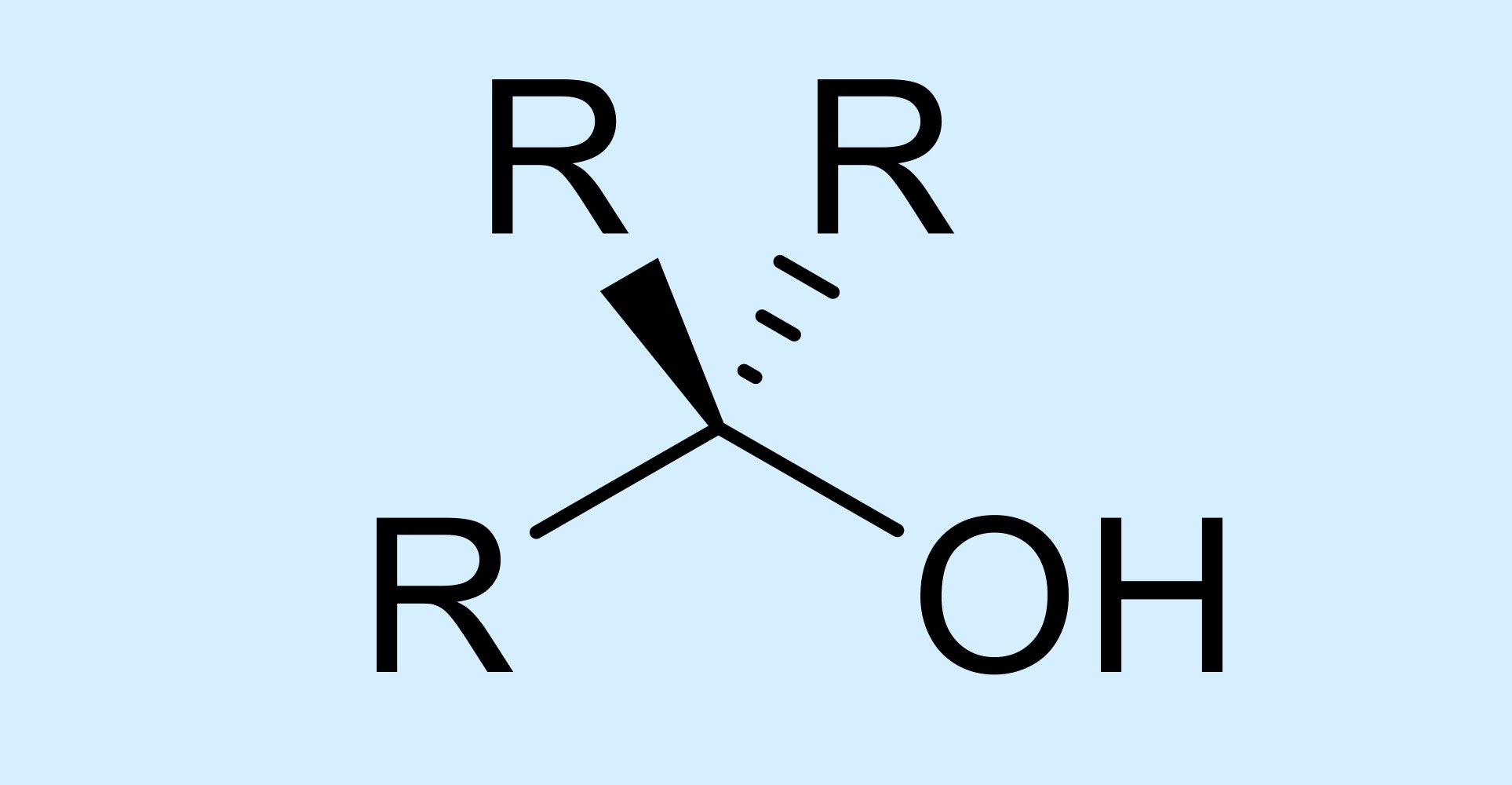
alcohol
phenol
hydroxyl attached to a benzene
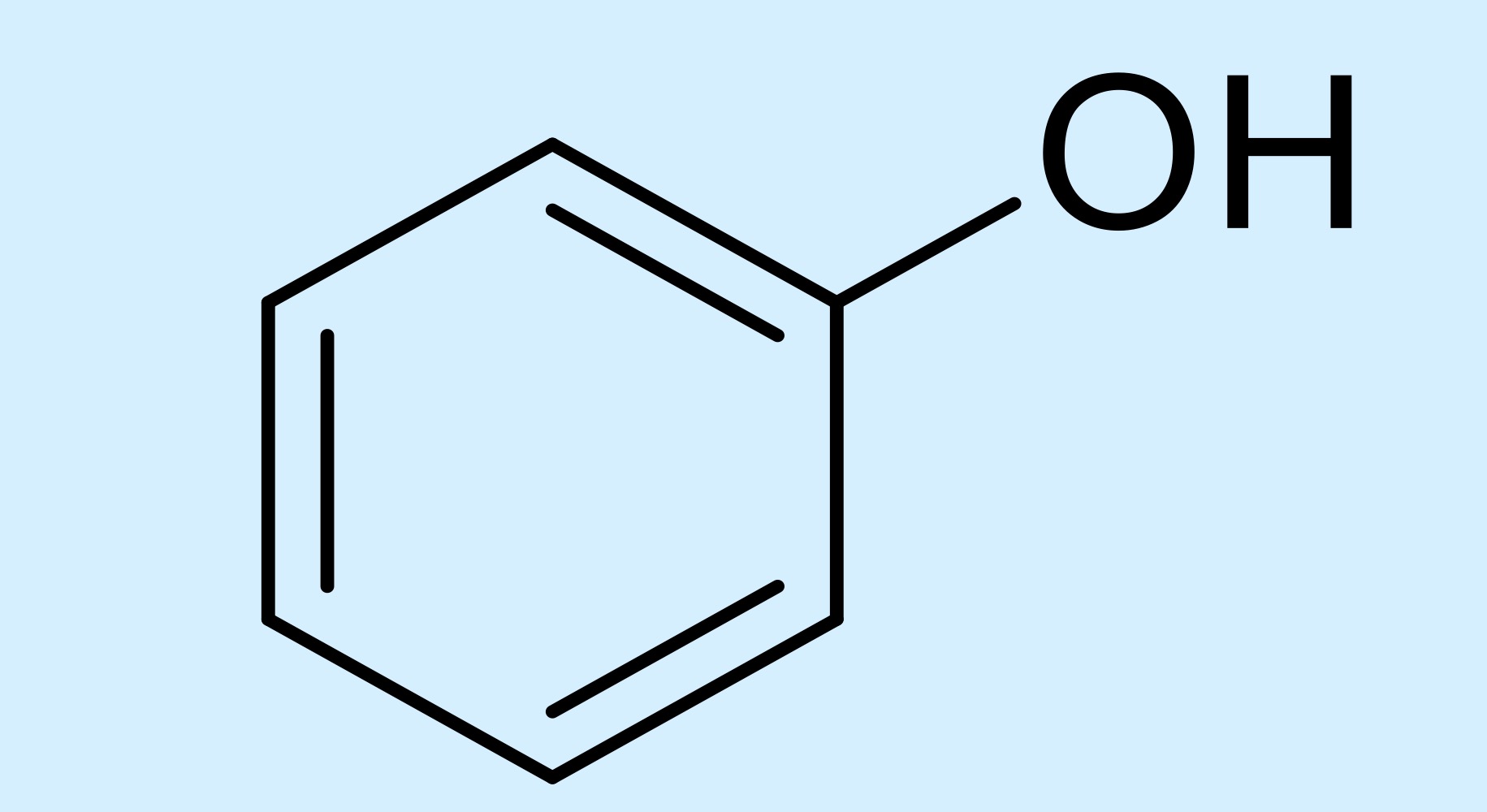
phenol
ether
an oxygen bonded to two carbon groups
epoxide
triangular carbon-carbon-oxygen ring
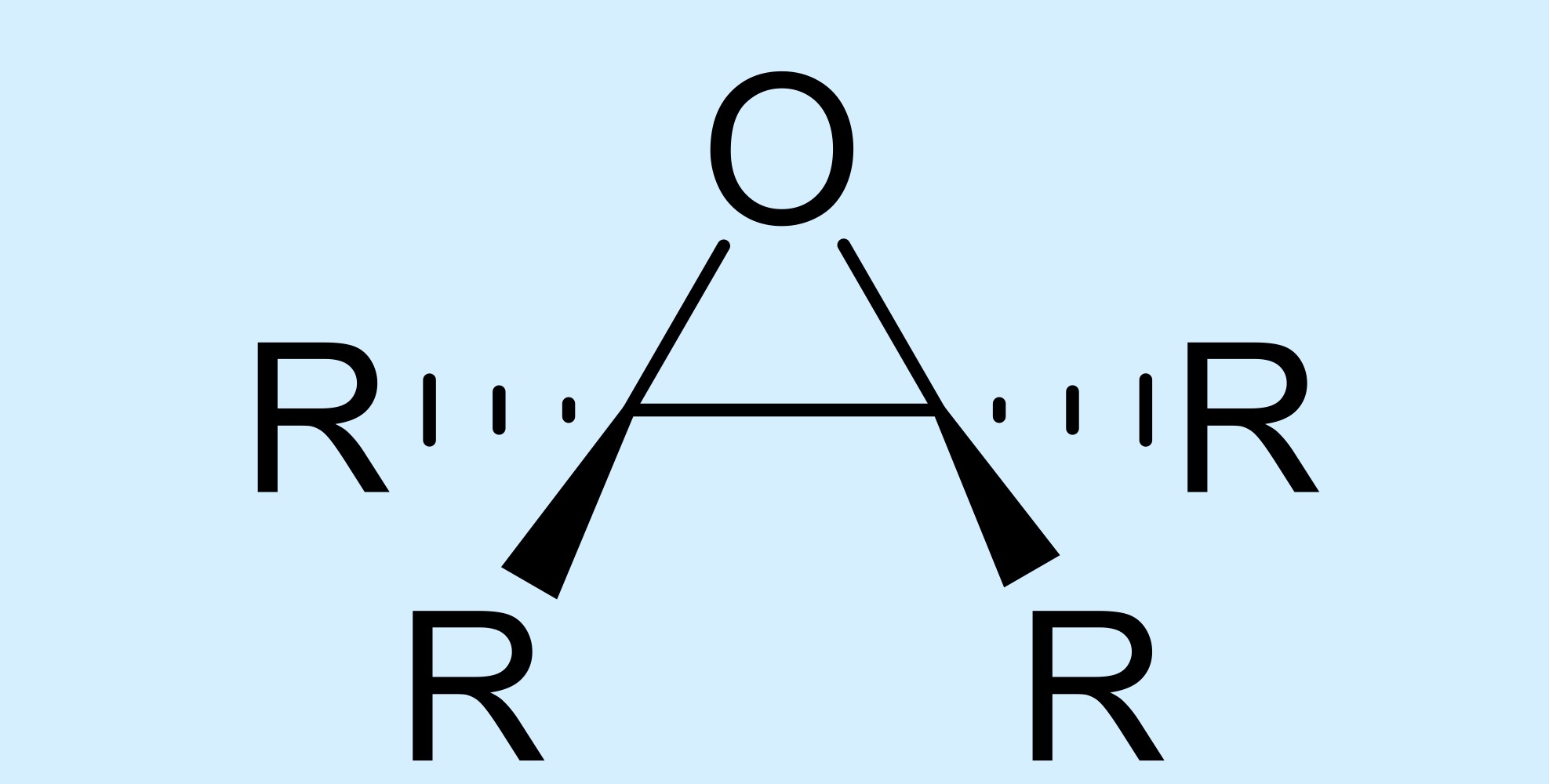
epoxide
alkanethiol
hydrocarbon chain with a -SH group at the end
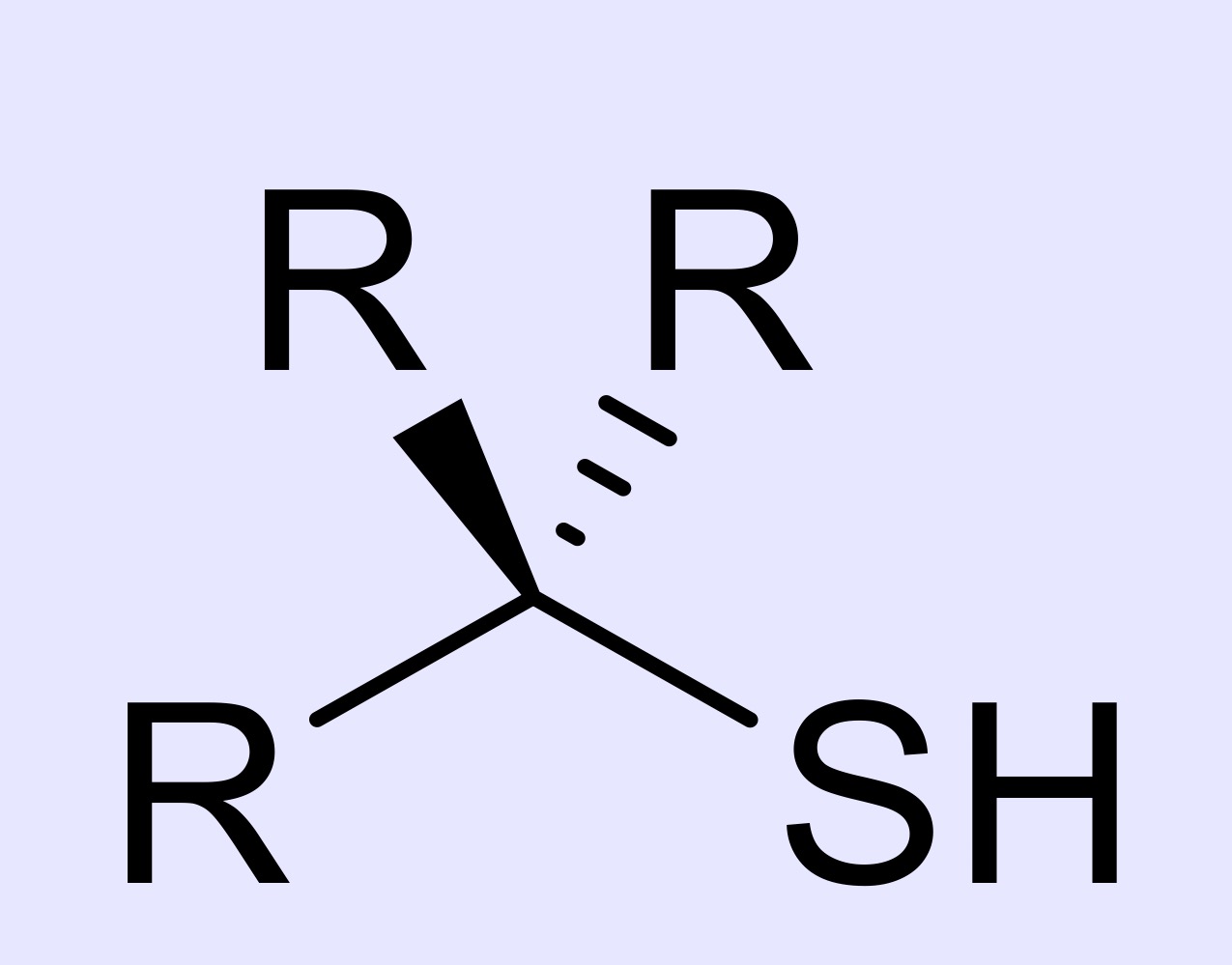
alkanethiol
arenethiol
-SH attached to a benzene ring
sulfide
S bridges two alkyls
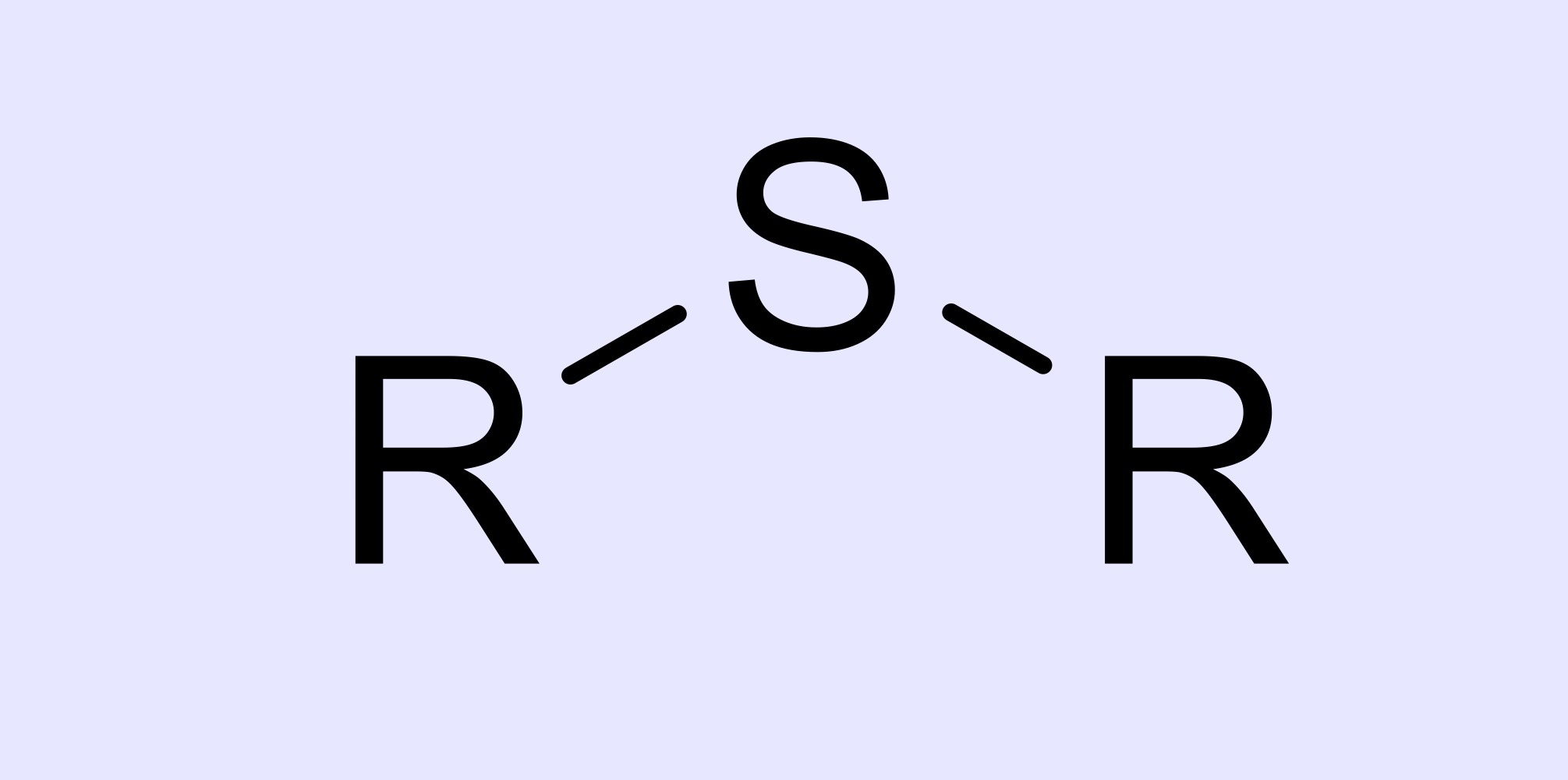
sulfide
haloalkane
alkane where one or more hydrogen atoms is replaced by a halogen
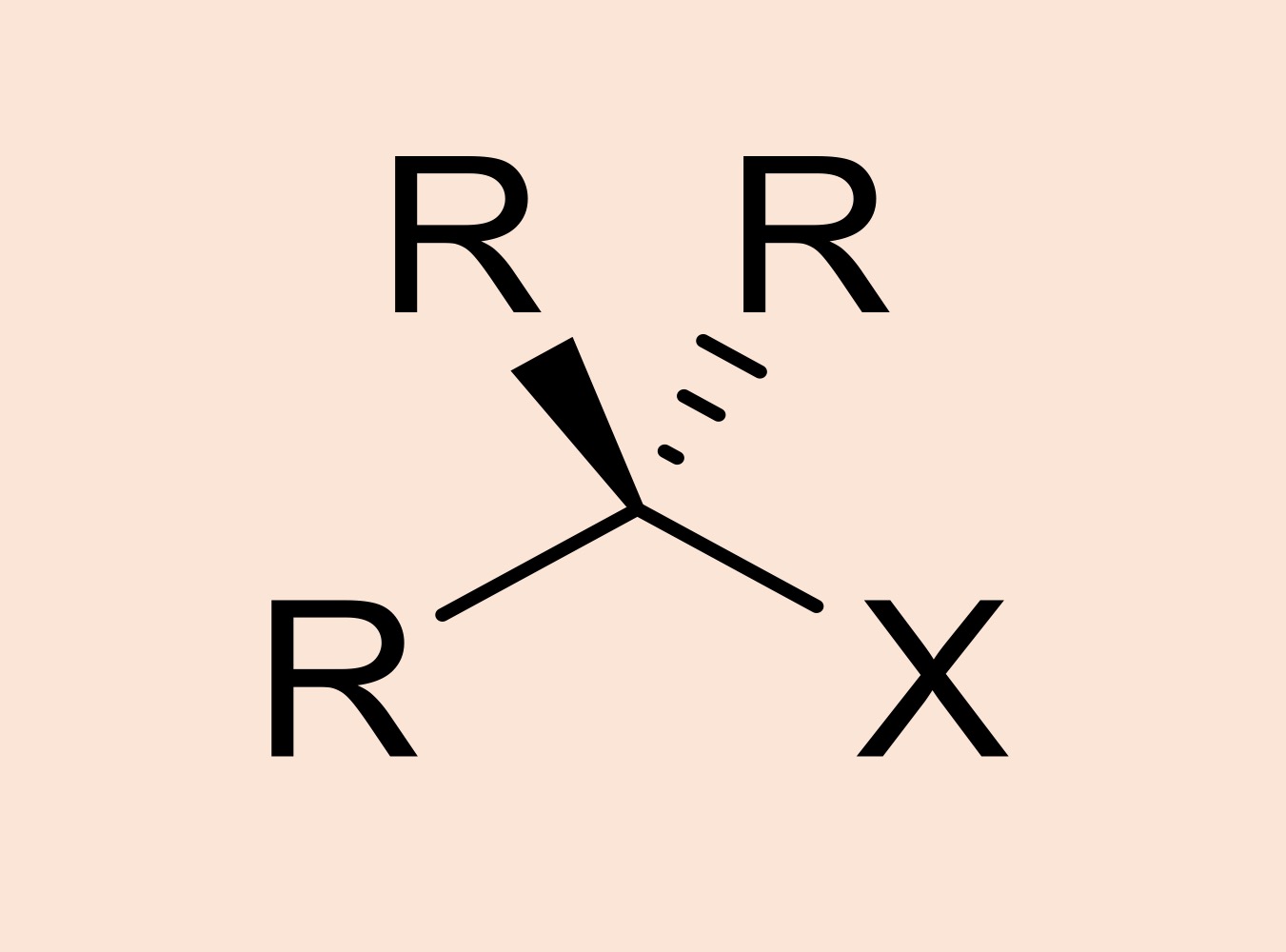
haloalkane
amine
N atom with three bonds, one or more being an alkyl and the rest being H
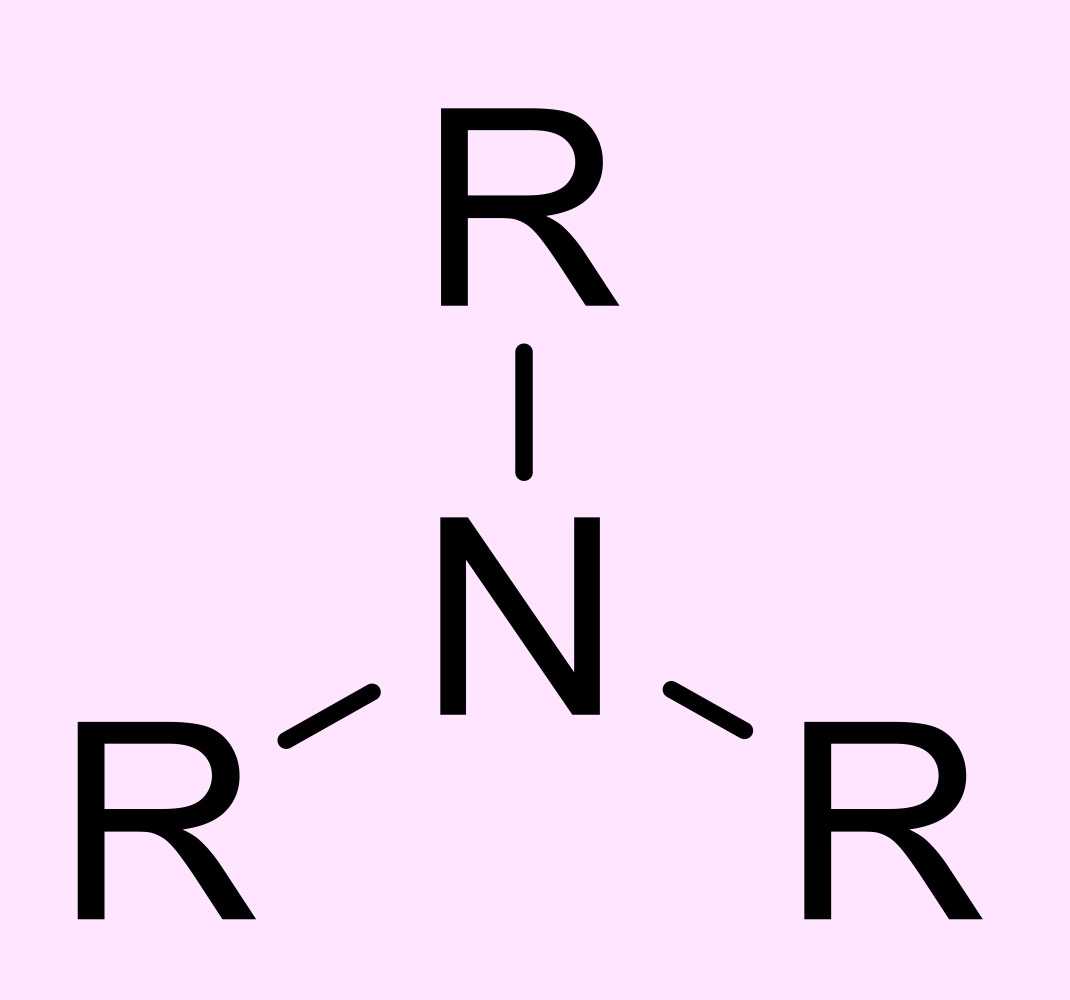
amine
nitrile
nitrogen triple bonded to a carbon
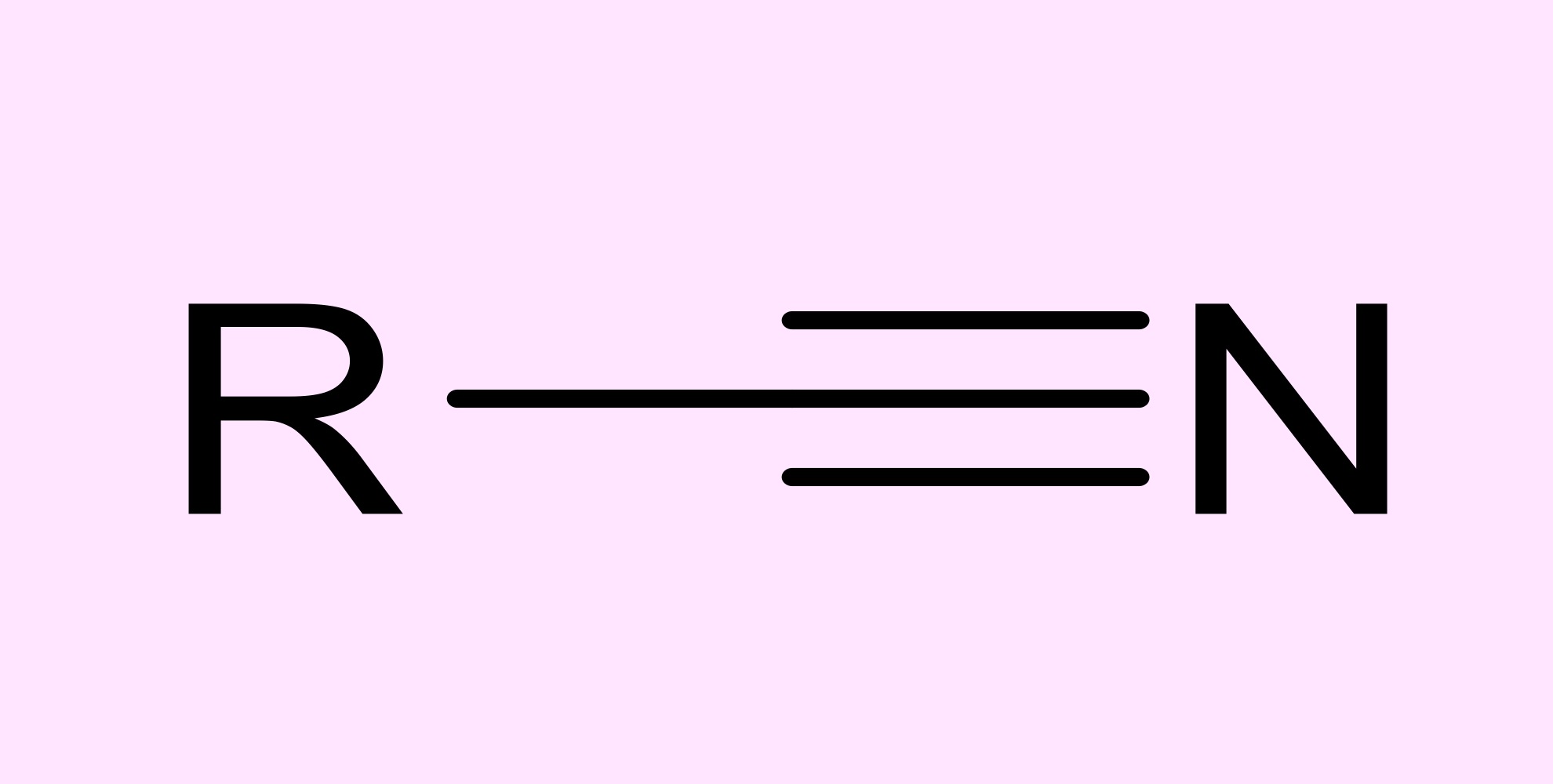
nitrile
aldehyde
carbon double bonded to an oxygen, single bonded to a hydrogen and alkyl
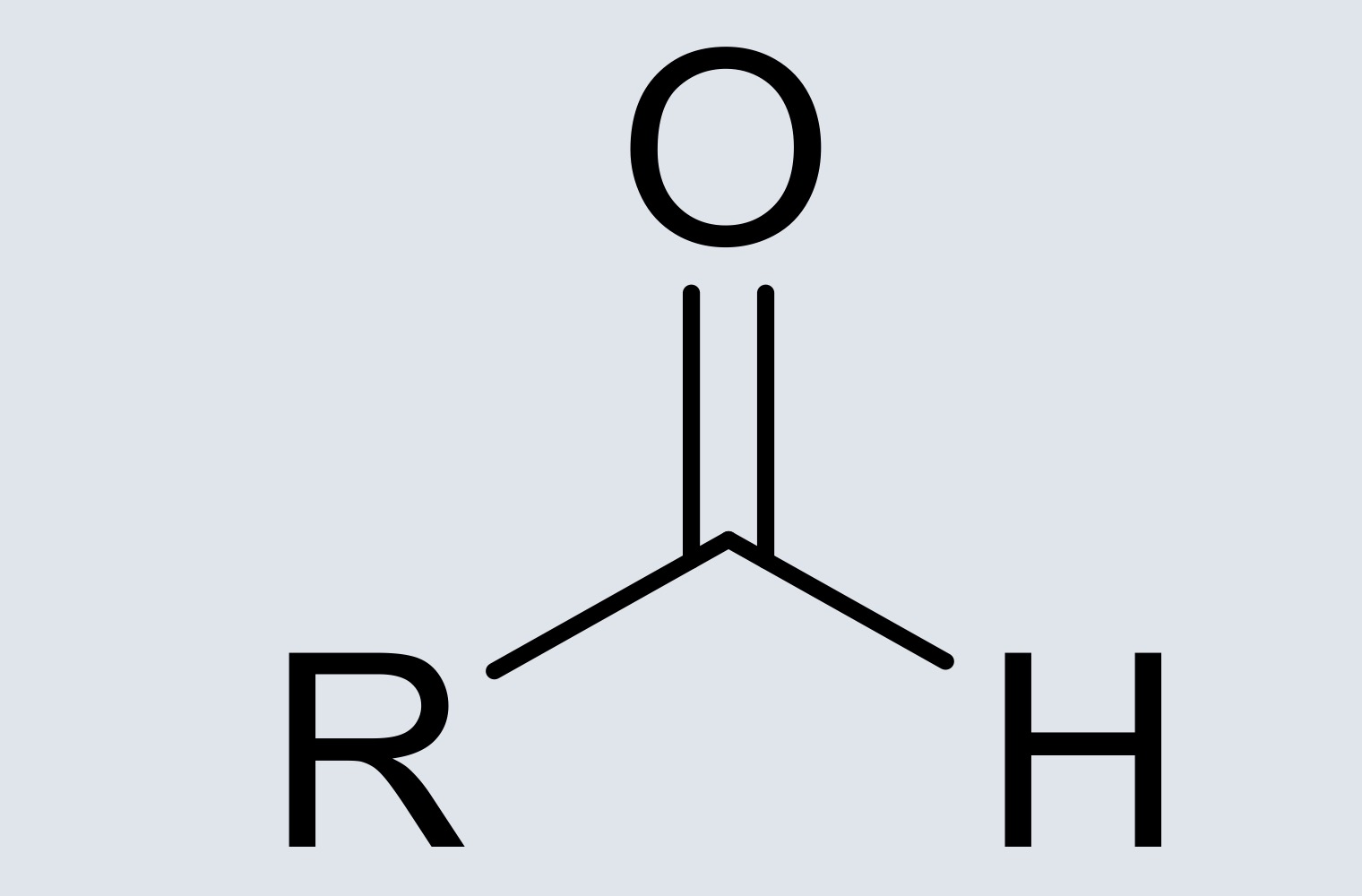
aldehyde
ketone
carbon bonded to two alkyls and double bonded to an oxygen
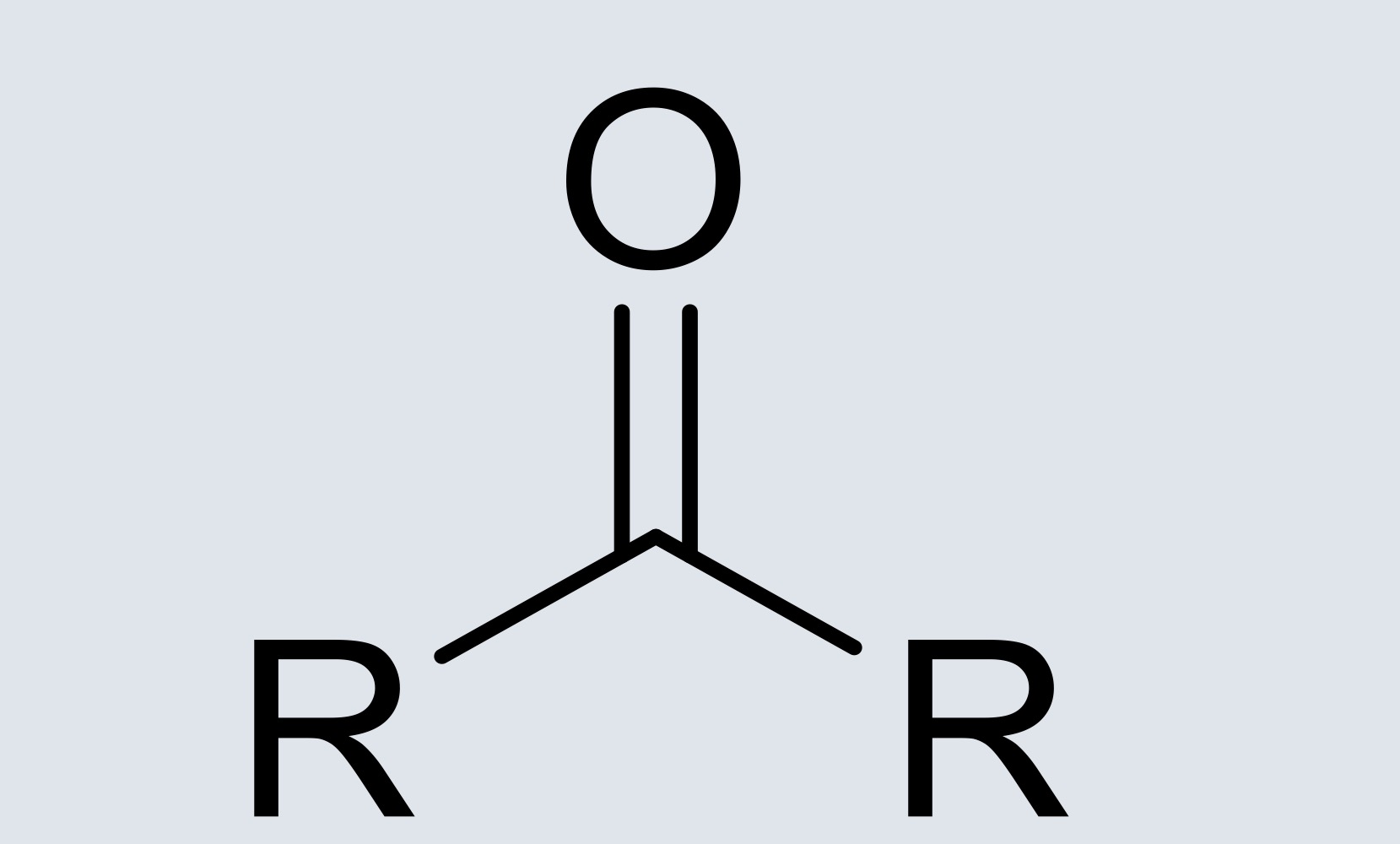
ketone
carboxylic acid
-COOH double bonded to an oxygen and single bonded to a hydroxyl and a hydrocarbon
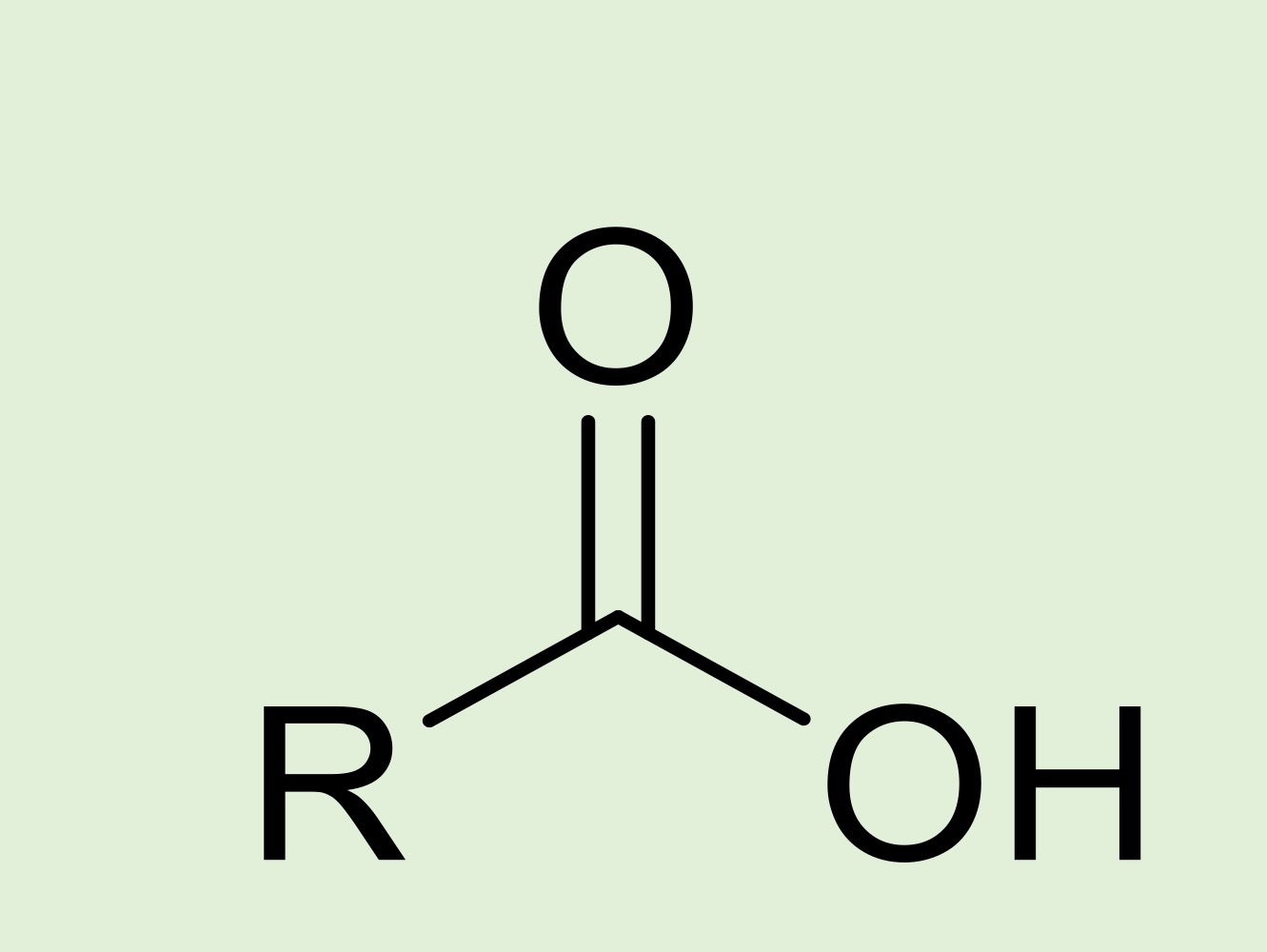
carboxylic acid
acid anahydride
two acyl groups (R-C=O) linked by an oxygen
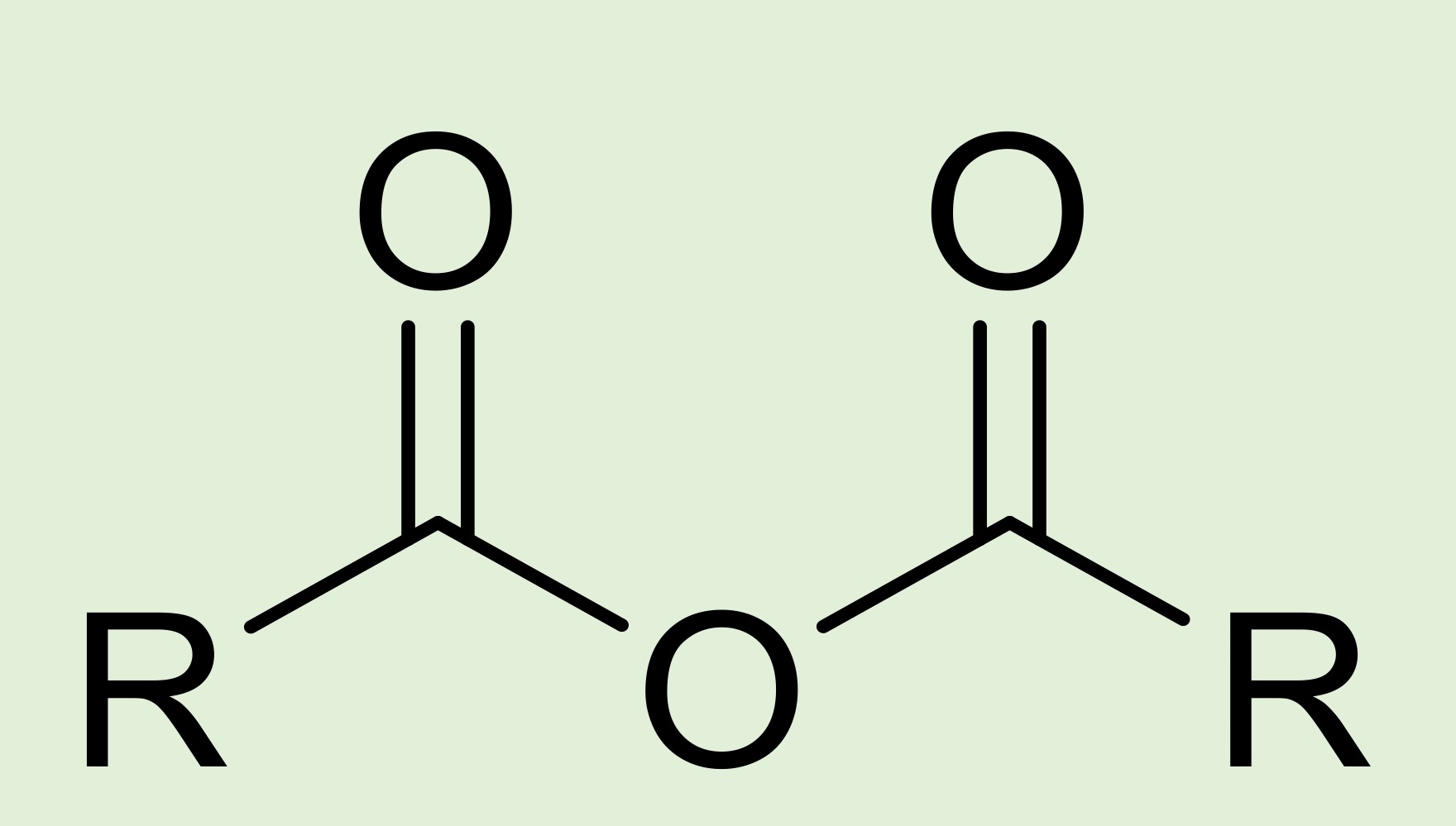
acid anahydride
ester
carbon double bonded to an oxygen, single bonded to a carbon chain and another oxygen which is then attached to another carbon chain
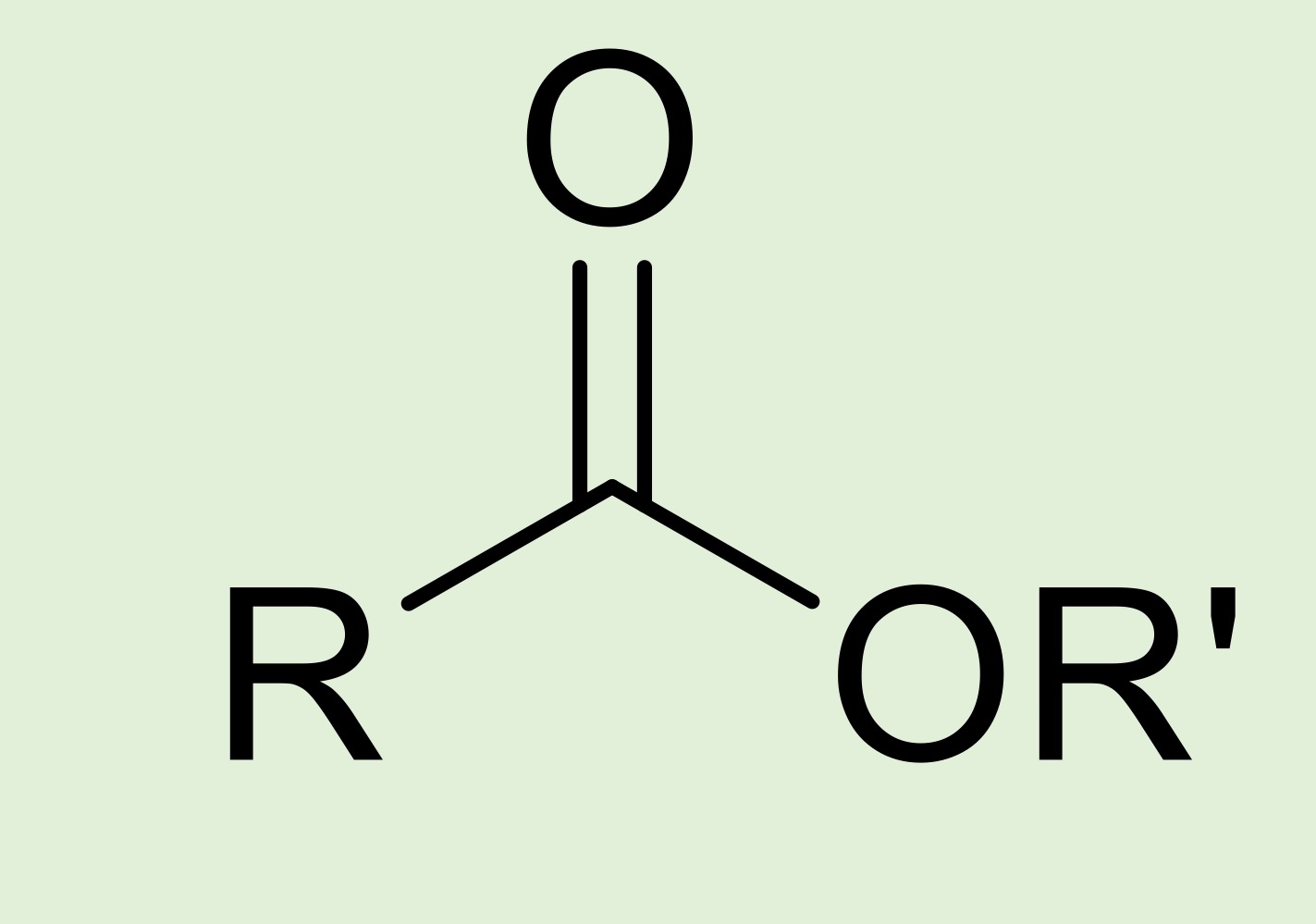
ester
acyl/acid halide
Carbon double bonded to an oxygen, R group, and a halogen
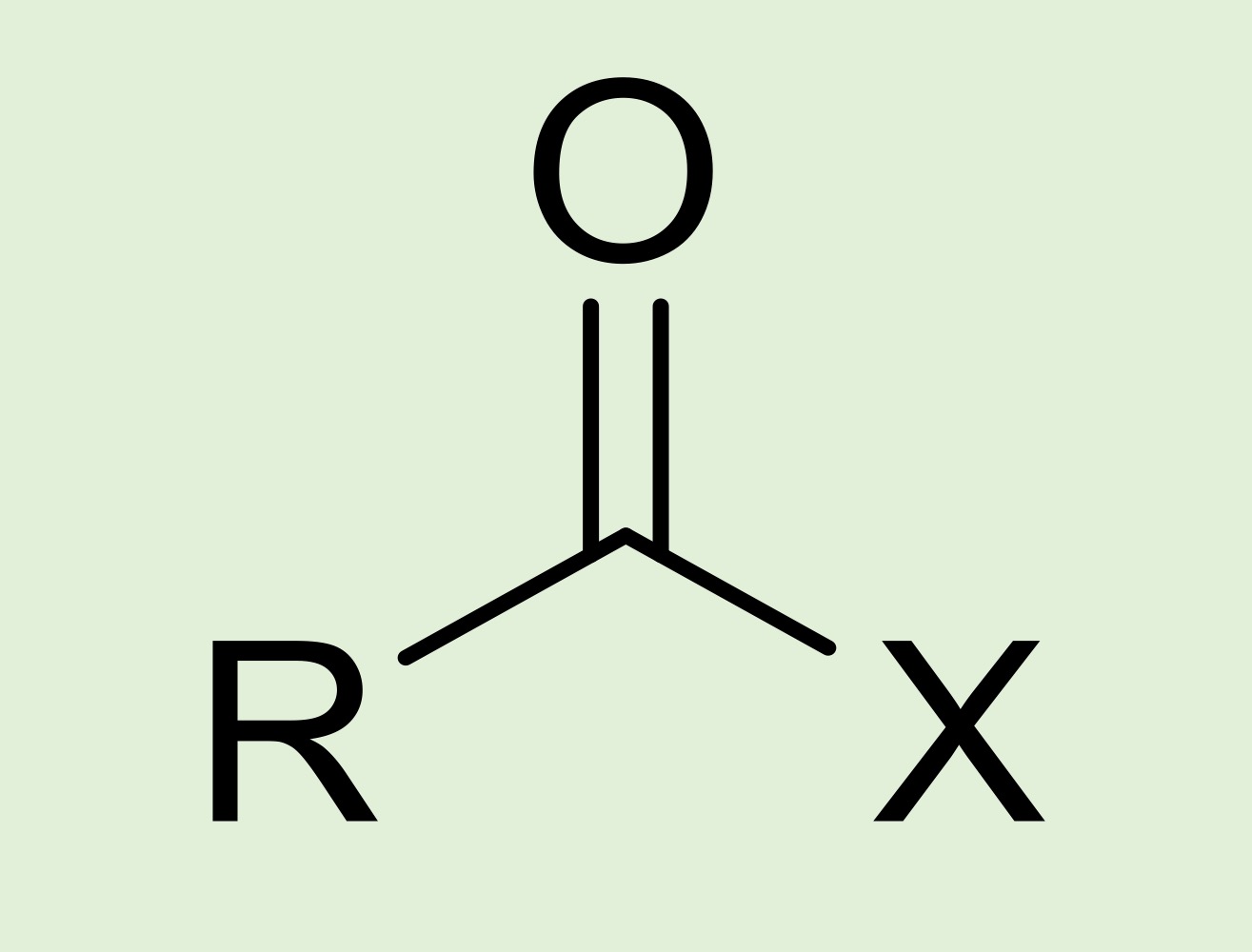
acyl/acid halide