rate of reaction experiment
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
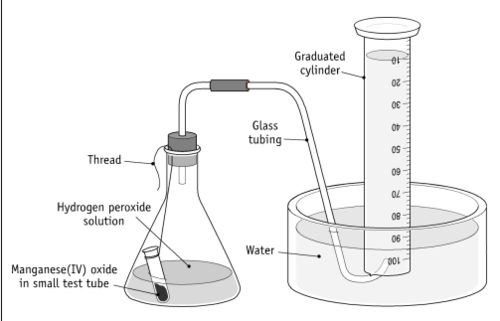
experiment set up
to monitor the rate of production of oxygen from hydrogen peroxide using mangones dioxide as a catalyst
chemical equation
H2O2 —MnO2—> H2O + 1/2O2
manganese dioxide
a black powder that acts as a catalyst to speed up the decomposition of the hydrogen peroxide
steps for the experiment
dilute hydrogen peroxide is placed in a conical flask
delivery tube is connected to an inverted graduated cylinder filled with water and standing on a basin of water
a small amount of manganese dioxide is quickly added to the hydrogen peroxide in conical flask and it is stoppered immediately. shake the conical flask and start the timer
the volume of gas (oxygen) in the graduated cylinder is measured at regular intervals
graphing
oxygen: y axis
time: x axis
average rate
total volume of oxygen given off (cm3)
divided by total time for reaction to go to completion (min)