Chemistry s2.2 and s3.2
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Alcohol
Hydroxyl functional group
-anol
CnH2n+1 OH

Alkane
Single carbon bond
-ane
Cn H 2n+2
Alkene
Double carbon bond
ene ending
Alkenyl functional group
Cn
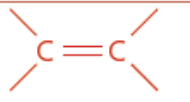
Alkyne
Triple carbon bond
-yne ending
Alkynyl functional group
Cn H 2n-2

Ether
Ether functional group
Isomer of alcohol
Oxyalkane ending
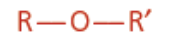
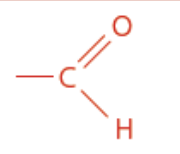
Aldehyde
Aldehyde/carbonyl functional group
Anal ending
Isomer of ketone
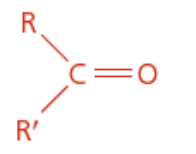
Ketone
Carbonyl functional group
Isomer of aldehyde
Anone ending
Carboxylic acid
Isomer of ester
Carboxyl functional group
Ends in anoic acid
CnH2n+1COOH
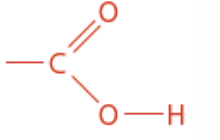
Ester
Isomer of carboxylic acid
Ester functional group
Ends in anoate
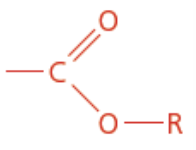
Amide
Nitrogen containing
Carboxyamide functional group
Ends in anamide
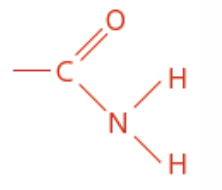
Amine
Nitrogen containing
Amine functional group
Ends in anamine

Nitrile
Nitrile functional group
Nitrogen containing
Ends in anenitrile

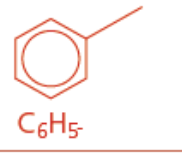
Arene/aromatic
Phenyl functional group
Ends in benzene
Halogenalkane
Halogeno functional group
Fluoro, chloro, bromo, iodo prefix
Ane suffix
When does ionic bonding occur (what electronegativity difference
If the difference in electronegativity between two elements is ≥ 1.8
Covalent bonding
The electrostatic attraction between a pair of electrons and positively charged nucleus.
Dative/co ordinate bond
• a type of covalent bond between two atoms in which the bonding electrons are supplied by one of the two atoms.
Formal charge formula
FC=no valence e - ½ bonding e - lone pair e
Polar or non polar covalent
If the difference si approximately greater than 0.5 the bond has some degree of polarity.
Can sometimes cancel out based on symmetrical shape
Differences of less than 0.4 are considered to be nonpolar.
VSEPR
See sheet practise with far right column
Van der waals
London dispersion forces
- Dipole - Dipole forces/interaction
Explain London dispersion forces
These exist due to a temporary dipole on a molecule.
Even in non-polar molecules electrons can at any one moment be unevenly distributed, this instantaneous dipole can cause or induce another dipole in a neighbouring molecule.
The result is a weak attraction - London Dispersion Force.
Increase in mass ( more electrons) = increase in London dispersion forces
All substances have them just to greater degrees
Dipole dipole interactions
These occur due to electrostatic attraction between molecules with permanent dipoles.
Strength increases with increasing polarity
Usually when NOF and the Halogens are present but for all non symetrical polar molecules
Hydrogen bonding
This occurs when hydrogen is bonded directly to a small, highly electronegative element.
e.g. N,O,F
The electronegative atom draws the covalent bonding pair of electrons away from the Hydrogen atom. The proton in the nucleus of the Hydrogen atom attracts the non-bonding electrons in N, O or F on neighbouring molecules.
A strong Dipole attraction occurs.
● σ Sigma bond what it is and when it occurs
formed when two atomic orbitals on different atoms overlap along an imaginary line drawn through the two nuclei.
(the electron distribution has axial symmetry around the axis joining two nuclei)
● occurs when
s atomic orbitals overlap with s atomic orbitals
s atomic orbitals overlap with p atomic orbitals
p atomic orbitals overlap with p atomic orbitals head on
Required for single and double and triple bonds (only once)
Pi bonds
Formed when two p orbitals overlap 'sideways' on.
○ (combination of parallel p orbitals)
● overlap occurs above and below the imaginary line drawn
through two nuclei
● One π bond is made up of two regions of electron density
In single bond once and triple bond 3x
Delocalization
In some molecules bonding electrons are less restricted to one position, we say they are delocalised. They spread out giving greater stability.
Gives bond order 1.5 of Orginal
Resonance
Resonance structures occur when there is more than one valid Lewis representations for a multiple bond in a molecule. The actual species is therefore a hybrid of the two structures - delocalisation.
The electrons in the pi bond delocalise making the molecule more stable.
Diagram to represent delocalisation.
Benzene reasons for it
1- all bond lengths and angles measured to be the same - if double bonds were shorter, it wouldnt be perfectly hexagonal so electrons must be delocalized and resonance structure
2- ring of delocalized electrons makes benzene more stable than expected
3- doesnt undergo reactions exprected for molecule with double bonds (ussually in addition reaction with bromine will absorb and lose colour, but here it remains orange)
Sp3 hybridization
Draw
Single bonds
Eg methane
Tetrahedral
No pi bonds
Sp2 hybridization
Draw
Eg ethene
Double bonds
Trigonal planar
1 pi bond
Sp hybridization
Triple bonds
Eg ethyne
Linear
Draw and explain bond angle, number of bonds how many electrons promoted
2 pi bond
Allotropes of carbon sheet
Homologous series
family of compounds in which successive members differ by a common structural unit, typically CH2. Each homologous series can be described by a general formula.
CSF practise
Prefix eg meth eth
1 = Meth 2= Eth
3 = Prop 4 = But
5 = Pent 6 = Hex 7 = Hept 8 = Oct 9 = Non 10 = Dec
Saturated and unsaturated
Saturated compounds contain single bonds only and unsaturated compounds contain double or triple bonds.
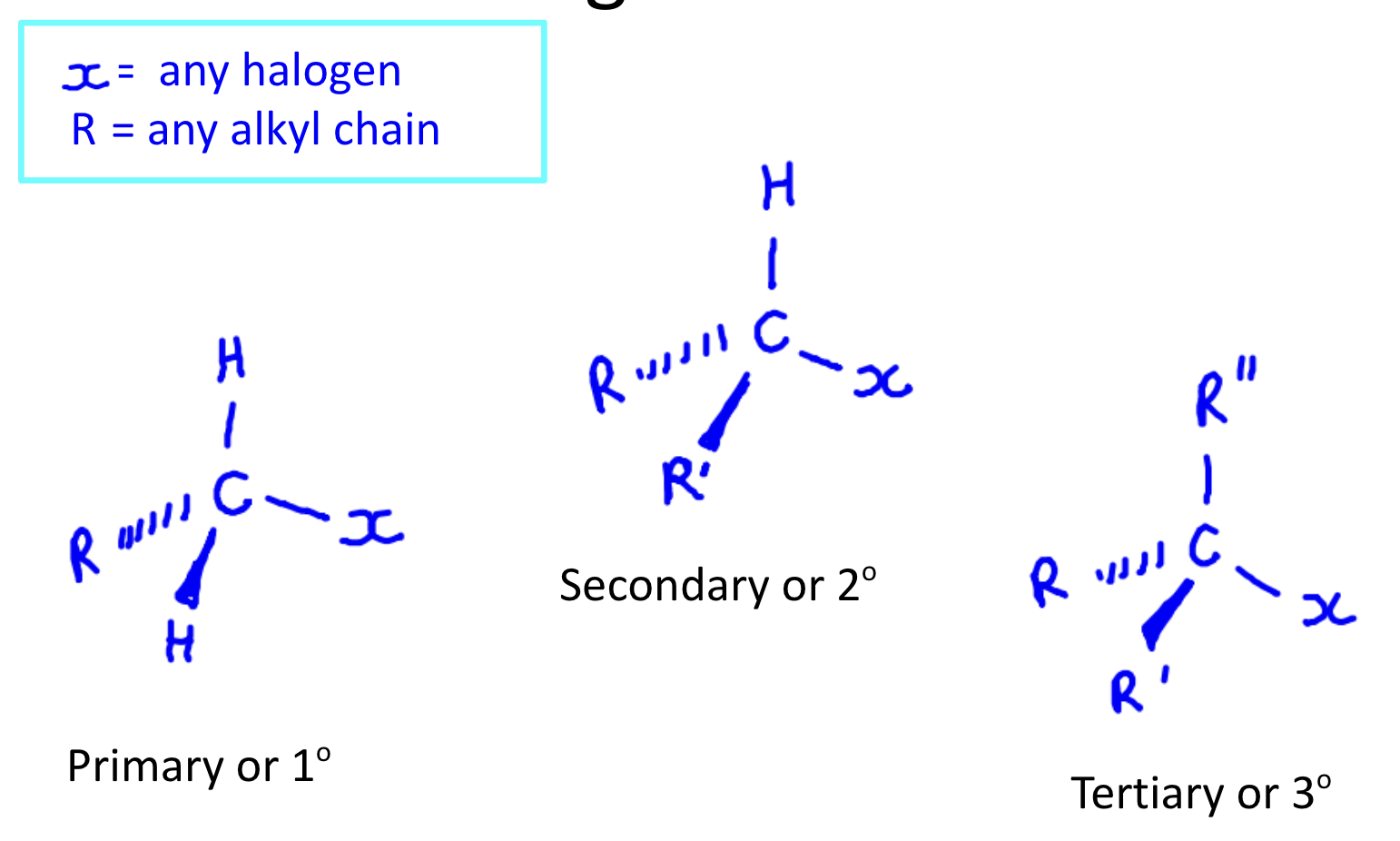
Primary secondary tertiary
Cis
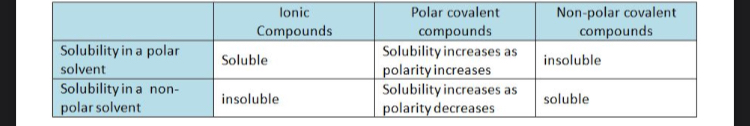
Solubility polar and non polar
Like dissolves like is simplified
As the non-polar section of a molecule increases, the molecule becomes less soluble in water.
Electrical conductivity polar and non polar
Covalent compounds do not contain ions therefore molecular substances aren't able to conduct electricity in the liquid or solid state.
Some polar covalent substances can ionise in certain conditions, these substances will then be able to conduct electricity.
polar
a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Paper chromatography
used to separate a mixture of solutes in a solvent. The mixture to be separated is first dissolved in a solvent.
The stationary phase is the water trapped between the cellulose fibres of the paper. The mobile phase is a developing solution that travels up the stationary phase, carrying the samples with it.
chromatography retardation factor
Rf = distance travelled by solute/distance travelled by solvent.
the distance travelled by solvent is where the water line ends and the distance travelled by solute is taken from the midpoint