IMOS Midterm Review
1/137
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
138 Terms
Properties of Clay
high plasticity (manipulated at room temp.)
good cohesion (maintains shape)
high strength under compression (after drying and baking)
poor tensile strength
opaque
insulator
can be dried
can be chemically transformed (heating above 1000C, shrinks, forms Si-O-Si bond)
Structure of clay
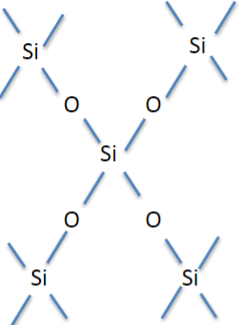
Si has 4 bonds (Si-O-Si)
Al has 6 bonds (Al-O-Al)
Oxygen has 2 covalent bonds (Si-O-Al)
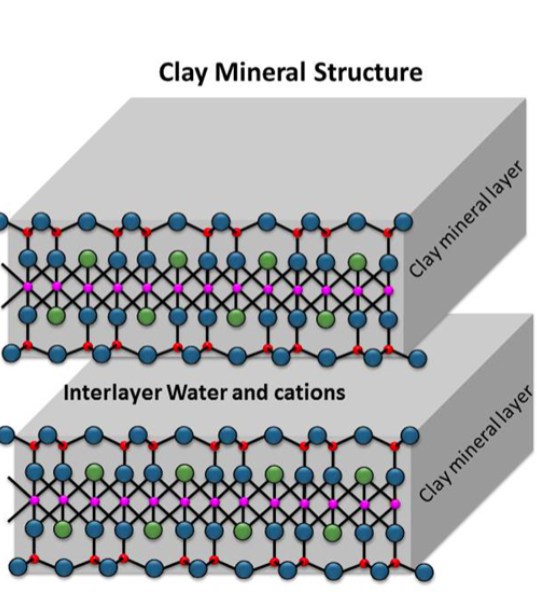
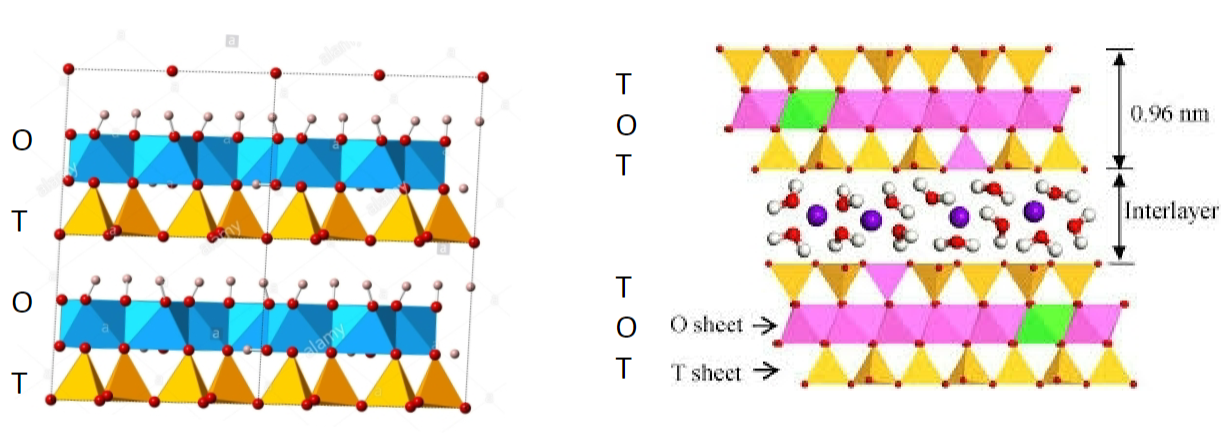
Kaolinite (China clay)
1:1 ratio (O-T-O-T)
lower shrink to swell capacity
heated <500C = drying can be reversed
heated >1000C = stoneware dishes
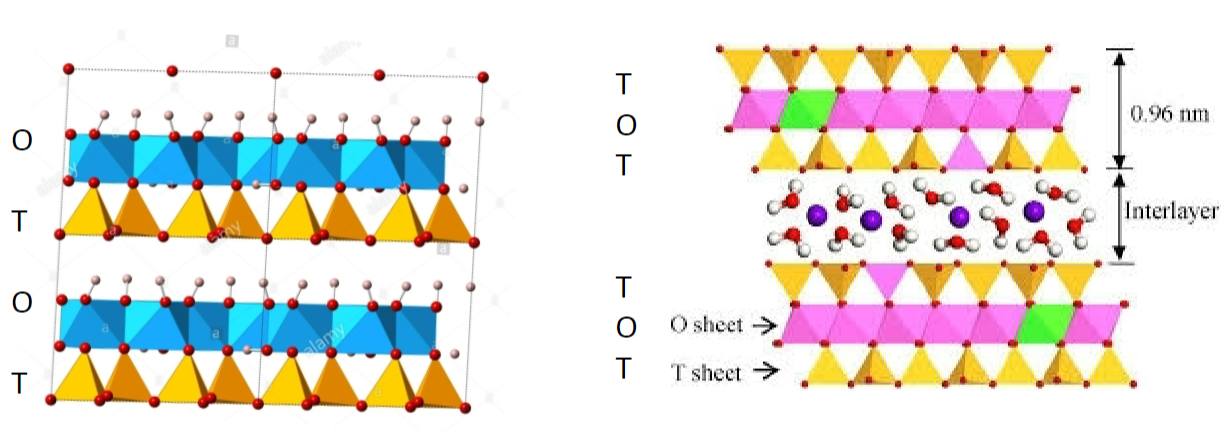
Montmorillonite
2:1 ratio (T-O-T-interlayer-T-O-T)
most common
large shrink to swell capacity
Temper
non-plastic materials used to prevent shrinkage and cracking during drying and firing of vesels (ex. bone, charcoal, wood ash, sand, crushed sandstone, crushed limestone, crushed volcanic rock, crushed shells)
Primary v. Secondary clays
primary: found at the site of formation
secondary: washed downstream
factors for types of clay
minerals
glaciation
ratio
Cuneiform
oldest written story (2700 BC)
account of King Ur’s superhuman strength and journey for immortality
influence Illiad and Odyssey
History of clay
Paleolithic: clay figurines
Mesolithic: Japanese hunter-gatherers used clay pots for cooking
Neolithic: sun-dried clay bricks in Israel, (oldest inhabited cities); crops stored in clay
7000 BC: Chatal Huyuk Clay Society
5000: potters wheel
4000BC: cuneiform tablets
Ancient uses of clay
building materials (Great Ziggurat of Ur)
writing
cooking
storage
sling ammunition
medical (Armenian bole medicine drink)
musical instruments (ocarina flute)
Modern uses of clay
sealing of oil drilling, landfills, and dams
building materials
odor absorbents
pottery
toothpaste
cosmetics
paint
gasoline production
papermaking
cement production
byproduct of phosphate mining
chemical filtering
organic farming
quick-clot combat gauze
hazardous waste clean-up
Carbon capture
contains olivine (MgSiO4)
traps Co2 into solid form
captures up to 1/3 of weight in CO2
could remove up to 1.7 trillions lbs of CO2 (5% of excess CO2 per year)
Affordances v. Constraints
affordances: durable, hard, watertight, thermal conductivity
constraints: brittle
Catalhoyuk clay society
mound settlement; entangled with clay
Theory of Entanglement
humans depend on things
things depend of things
things depend on humans (chains of interdependence)
humans depend of things that depend on humans/other things
Things
an assembling or bringing together of properties (potential and actualized)
Material entanglement
influences social and cultural traits over time
creates “thingworlds”
new materials are selected if they fit within existing entanglements
social change is not dependent on human intervention
Rare Earths
not easily smelted
hard to isolate
often found in clay
polluting to extract
chemically similar (Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, gd, Tb, Dy, Ho, Er, Tm, Yb)
Uses of rare earths
low-energy LEDs
strong magnets
wind turvines
electrical vehicles
polishing lgass
alloying agent for stell
phone LEDs
Extraction of rare earths
China extracts 63%
byproduct of steel production
restricted exports and caused a the price spike
removed export restrictions and dropped worldwide prices
source may not be sustanible (critical material)
Rare earth sustanibility
massive recycling effort
use less waste during extraction
Properties of Gold
color: gold, butter yellow
hardness: 2-3
element name: Au
very dense: gold m³ = 19,300 kg
most ductile
most malleable
can be optically transparent
does not oxidize
does not combine/react with other elements often
does not react with acids
good electrical conductor
Properties of Silver
color: silver-white
hardness: 2.5-3
very malleable
very ductile
oxidizes (changes color)
naturally occurring as chloride or sulfide
good electrical conductor
good thermal conductor
most lustrous metal
Gold v. Silver Properties
soft
conductive
currency metals
weak metallic bonds
extra electron
Method of Extraction
amalgamation
cyanidation
liquidation
slucing
Cupellation
Parting
Amalgamation
extracting gold from the rock by 1) dissolving crushed gold ore with mercury 2) boiling off mercury
Cyanidation
extracting gold rocks by dissolving gold in cyanide solution
high percentage of gold extraction
potentially toxic
Liquidation
extracting gold and silver from copper by 1) mixing with leab 2) heating until lead melts 3) pour off lead 4) following with cupellation
Slucing
extracting gold from graving using gravity (think sifting during goldrush)
Cupellation
extracting silver from lead by 1) heating lead sulfide 2) lead oxidizies at 960C 3) silver is left behind
1 ton of lead = 1 lb of silver
toxic lead pollutes waterways
Parting
separating gold and silver from each other by 1) heating with salt 2) forms silver chloride 3) turn into silver
Ancient History of Gold (40,000 BC-50 BC)
used in Egypt for burials
first coins are made in Asia Minor
Alexander the Great leads the largest military campaign sponsored by gold
Greeks and Jews in Alexandria practiced alchemy
Julius Caesar bring back gold from Gaul to repay Rome’s debts
Modern History of Gold (700 AD-1899 AD)
Gold is discovered in Brazil and becomes the largest producer
17 lb nugget is found in North Carolina
the first goldrush sparked in North Carolina
California Goldrush is triggered and hastens Western settlement
Gold is discovered in New South Wales, AUS
USS Central America sinks 30,000 lbs of gold near North Carolina coast
Gold and silver found in Nevada
Gold is found in South Africa and has become the majority of mining
Gold is discovered in salmon river and created the Klondike Goldrush
Contemporary History of Gold (1900-Present)
Engelhard Corp. introduces a way to print gold on surfaces
Niels Bohr, Mac Von Laue, and James Frank make discoveries using gold
George de Hevesy dissolved Nobel Prizes to hide from Nazis
USS Central America is discovered (only 20% of gold is recovered)
total Gold discovered = 187,000 tons = all from metors
Modern Uses of Gold
currency standard until WWII
used in jewlery and awards
usefil in microelectronic industry
used for decorative purposes
used for red coloring in glass
used as reflector (space face shields)
used in cockpits
Modern Uses of Silver
used in decorative arts
used in photography and photographic firm
used as a conductor of electricity and heat
used for coins (90% of coin)
used for jewelry and silverware
Reasons to Use Alloys
keeps costs down
makes gold stronger
changes color
Karat System
24 kt = 100% (very soft)
18 kt = 75%
14 kt = 58%
12 kt = 50%
10 kt = 42%
<10 kt = not gold in US
Intrisic v. Extrinisc Value
Intrinsic: naturally occurring value
Extrinsic: value deemed by society (use in technology, medicine, electronics)
Money v. Currency v. Coins
money: medium of exchange
currency: a form of money guaranteed by a territory or government
coins: a form of money and currency
Roles of Money
medium of exchange
standard of value (comparison of goods)
way of storing value
means of payment
Characteristics of Money
durability
divisibility
portability
homogeneity
acceptability
limited/stable supply
History of Gold/Silver as Money
precious metals described as wealth in the Old Testament
first record in cuneiform tablet
first form as hack-silver
first datable coins from Ephesus (electrum coins = less gold = scam)
Ancient Egypt: kites of silver
Ancient Greek: drachma and electrum coin
India: silver-punched coin
China: knife money and bronze spade-shaped coin
Nanotech Size
1 billionth of a meter
Modern Uses of Nanoparticles
cars
iPod
computers
washing machines
clothing
Nanoparticles and Interaction with Light
small size changes color and interaction with light
25 nm = red
50 nm = green
100 nm = orange
Pasmon Resonance
changing the size of particles changes how they interact with sound
Gold Nanoparticles
gold core and silica shell
absorb light in infared wavelengths
used in thermal ablation of tissue
tissue absorption is minimal
penetration is optimal
Uses for Nanomaterials in Medicine
drug delivery
chemotherapeutics
hyperthermia
therapeutic agent
carrying of imaging agent
release of drugs
Methods of Formation of Nanomaterials
colvalent organic sythesis
self-assembly
crystal formatino
laser ablation
grinding
milling
fabrication
Cement v. concrete
cement is binder for concrete
concrete is the composite (clinker + sand + rock)
Clinker process
<700C = water is lost
700-900C = calcination (CaCO3 = CaO + CO2)
1150-1200C = liquid phase
1250-1450C = clinker nodules form
after cooling = add gypsum to control setting
clinker is ground into Portlad Cement
Bitumen
various mixtures of hydrocarbons with nonmetallic derivatives that occur naturally after heat-refining natural substances
Properties of Bitumen
polymeric material (carbon, nitrogen, oxygen, hydrogen, sulfur)
not a unique molecular composition
found as natural surface deposits
easily collected
easily processed
compositionally stable at <300F
Uses for Bitumen
applied as coating/adhesive
used for paving and roofing
Advantages of concrete
can be mixed offsite
cures at room temp.
moldable into any shape
cheap
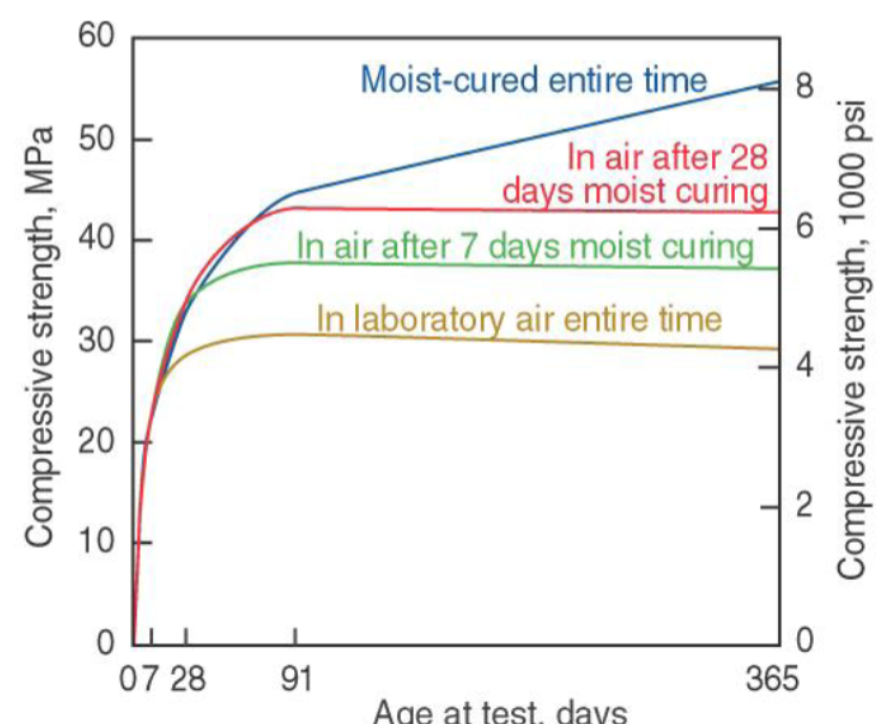
Disadvantages of concrete
relative durability
CO2 emission (7% of worldwide release)
1 ton of concrete = 1 ton of CO2
Future for concrete
use non-fossil fuels for heating (solar power)
green cement uses recycled material
zerocrete uses 100% recycled Flyash based cement
self-healing concrete uses bacteria and calcium lactate and heal cracks
Steel v. concrete
compression: good v. good
tension: good v. bad
thermal expansion coefficient is the same
alkaline environment reduces corrosion
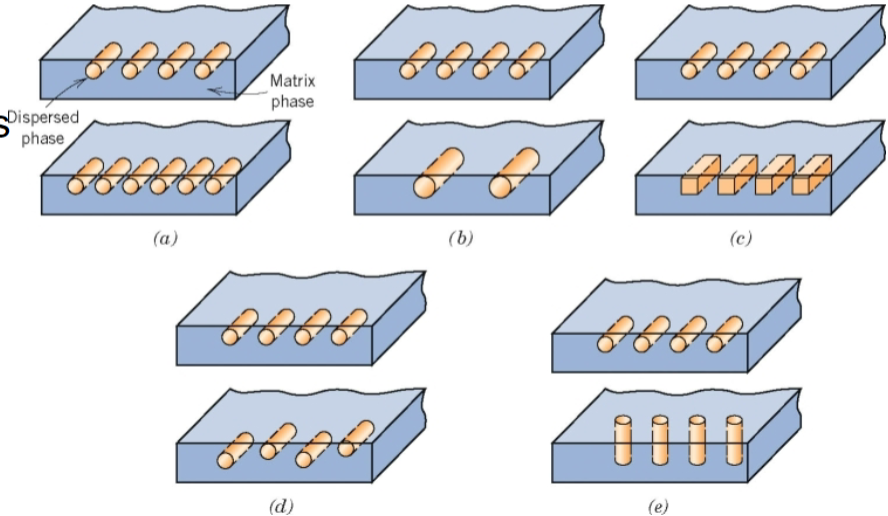
Composites
a material constructed from two or more components in greater physical or chemical properties are derived from the combination of compositions or structures
Properties of composites
componenets remain physically seperated
not naturally occuring
matrix and dispered phase
dependent on concentrartion, size, shape, distribution, orientation
concrete history
Ancient Greece used lime and pebbles
Romans use Roman concrete
Roman Empire falls and roman concrete recipe is lost
Middle Ages concrete is weak
John Smeaton discovers how to make cement hydraulic
USA uses cement for the Erie Canal
Portland Cement is invented
reinforced concrete is invented
first reinfoced concrete bridge is built in San Fransisco
Portland Cemenet
C + A + S + H + water + sand + rock
Roman concrete
used for compression structures (cantenary)
used different varieties for the dome of Parthenon
used for roads, buildings, aqueducts
sets underwater
self-healing properties
Roman concrete composition
quick lime formed from limestone and heat (CaCO3 = CaO + CO2)
fine pozzolana (volcanic ash replaces sand (Al2O3 + SiO2)
Water (H2O)
C+ A + S + H
Greek v. Roman architecture
doric
ionic
corinthian
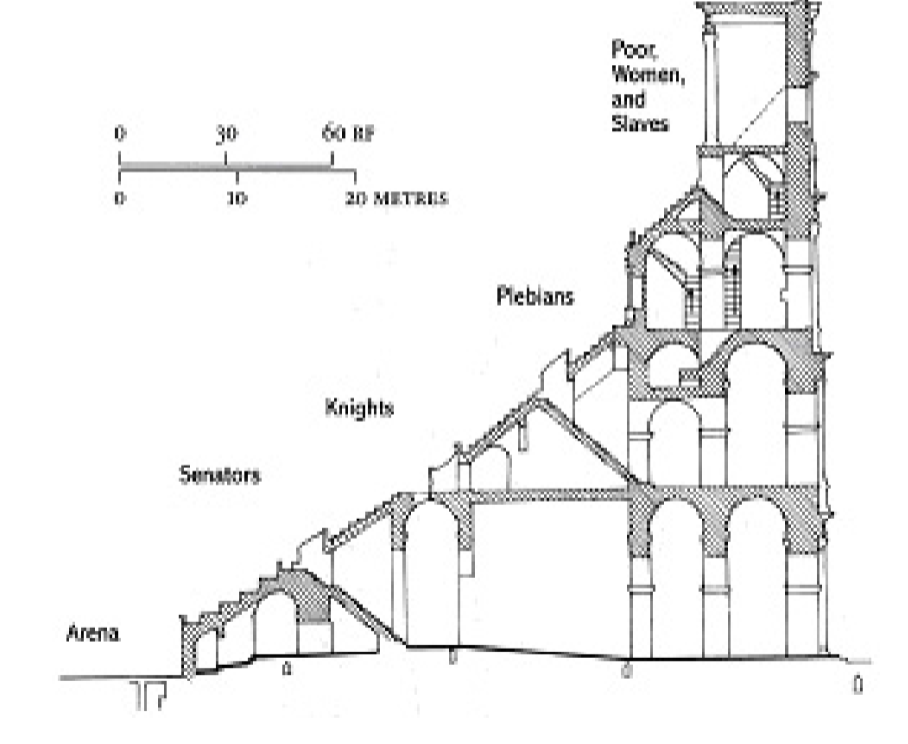
Class order of Romans
colessium seating reinforces class order
Properties of Copper and Bronze
metals
malleable
opaque
electrical conductor
thermall conductor
shiny, hard appearance
Copper v. Bronze
pure element v. alloy of copper and impurity (arsenic or tin)
melting point at 1084C v. 950C
hardness of 80 MPa v. 700 MPa
yield strength of 70 MPa v. 220 MPa
methods to strengthen bronze
plastic deformation (permanent)
elastic deformation (temporary) (dislocations → plastic deformation)
work hardening (add dislocations)
cold-rolling (increase tensile strength)
impurtities (block disolations)
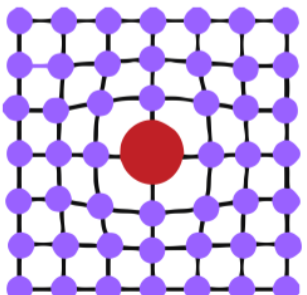
Copper reaction
CuCO3 + heat = 2Cu + CO2
2CuCo2 + O2 = 2CO
CuO + CO = Cu + CO2
Smelting process
calls for mixing of ore with charchoal
resource intensive
140 lbs of wood = 20 lbs of charchol
2 lbs of CuCo2 (malachite) = 1lb copper
crucible and furnace
Phase diagram of Arsenic
x-axis = composition (As)
y-axis = temp.
6.8% at 685C is max solubility in copper
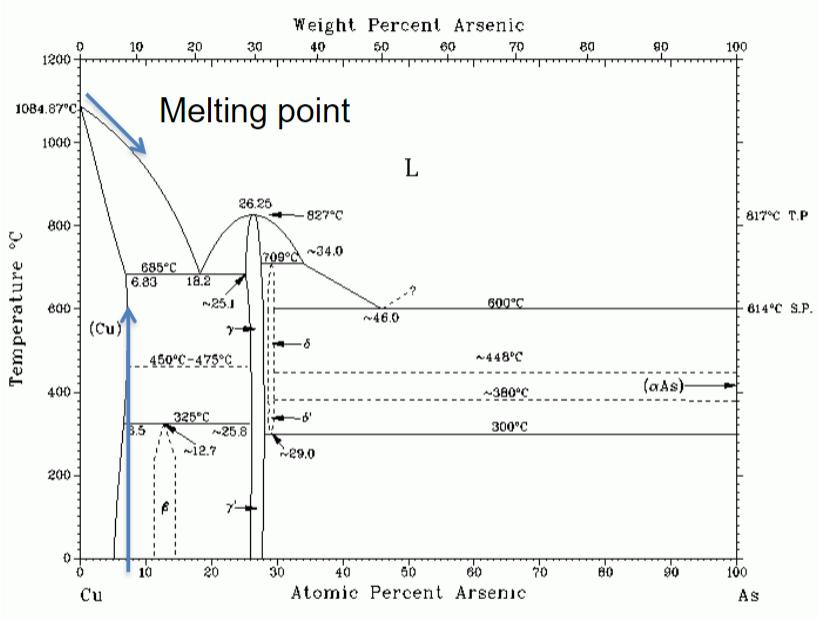
Arsenic alloy
produces altered color
increases the hardness of copper
lower melting point
oxidizes during smelting
produces AsO3 bi-product (toxic)
Tin alloy
increases strength
heat treat ment changes strength
adding too much tin can cause copper to be weaker
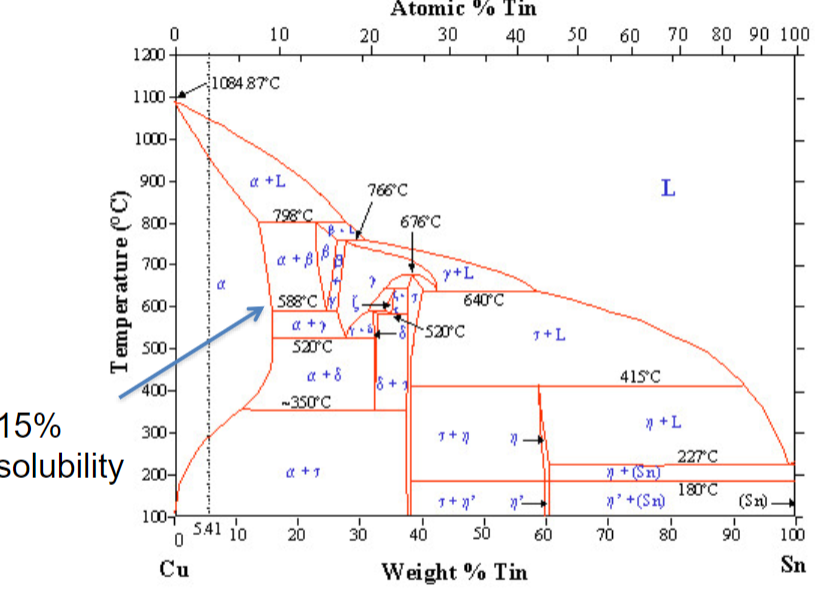
Tin v. Arsenic alloy
dissolve more tin than arsenic (<15%)
less toxic
harder to find than arsenic
Copper Age
5500-3000 BC
coppersmiths drew correlations between sources, conditions, and properties of copper
deposits’ purity depends on the region
used arsenic and tin as impurities
Bronze Age
3300-1200 BC
process of smelting
Otzi mummy is proof of copper smelting
King Solomon’s temple
parallel discoveries of copper around the world
social impacts (health, mining, exploration, and experimentation)
Trade developed around copper
England was the source of tin for Europe
Afghanistan also developed
casting technology created productino of art, tools, and weapons
Trade of Bronze Age
Egypt had gold
Afghanistan had tin (tin was precious metal)
Turkey had good metallurgists
very entangled society
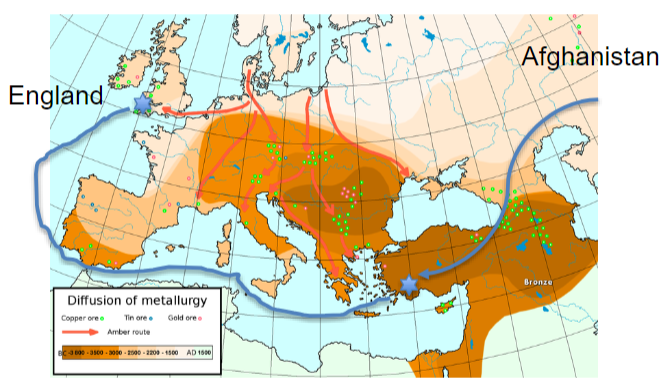
End of Bronze Age
200 yrs. of drought, mass starvation, and earthquakes
ports shut down, centers are not used, and the tin supply chain stops
sea people attack Egypt and Hittites
society breaks down
iron and Phencians rise
Annealing
cold treatment (less brittle, more efficient)
Copper ingots
circular shaped (England); ox-hide shaped (Mediterranean)
Copper ores
mMalachite (oxidized), Fahlerz (sulphides)
Pure Copper v. 10% Tin Copper Alloy
melting point 1083C v. 1000C
cast hardness: 50 HB v. 100 HB
cold-worked hardness: 100 HB v. 230 HB
Metallurgy importance
technological: new skills became necessary and common
economic: raw and finished materials became abundant
social: introduces new scales of value and social divisions
Coppersmiths
Germany
Spain
mythological smiths (Dwarfs)
Solar cells/Photovoltaic material
something that converts light into electricity; needs to beable to break bonds and create electrons and holes
Impacts of photovoltaics and solar cells
50% of CO2 comes from electricity generation
50-70% of the world relies of fossil fuels
solar energy produces 50x less CO2 than fossil fuels
solar energy is 300x cheaper than 40 years ago
industry growth is making solar energy cheaper
some regions need >9% of land area to produce enough energy
hard to recycle Si at the end of life
Grid parity
new energy cost = grid cost
Semiconductor materials
Si
CdTe
CIGS
Pervoskites
Properties of Ceramics
inorganic, nonmetal solid
brittle
high melting point
poor conductor
waterproof
chemically resistant (iconic and colvalent bonds)
crystallinity
Crystallinity
varies from crystalline to semi-crystalline to amorphous
Crystalline v. Amorphous
crystalline: long-range structural order
amorphous: lacking long-range structural order (ex. oxides, borides, polymers, and metals)
Vitrification
transformation of atomic/molecular structure
requires >1200C (for clay)
Plasticity
water layers can slide pass each other
Properties of Glass
inorganic, nonmetallic solid
brittle
high melting point
poor conductor
stoff
waterproof
chemically resistant (ionic and colvalent bonds)
amorphous (no crystallinity)
Operational Sequence
accounting an accounting of a system's procedures for start-up and shut-down, response to varying conditions, and certain scheduled operations
Operational sequence v. entanglement
operation sequence: steps of a process to make a thing
entanglement: how things and people are dependent on each other
Flinknapping
controlled reduction of glass-like rock (ex. fluted Clovis points)
requires the right kind of rock
skills for applying controlled force
produce flakes and blades with/ sharp edges
an infinite variety of forms
Bifacial reduction
planned reduction of the core through successive function or applications