MII - Malaria y
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What parasite species causes malaria?
Plasmodium species.
Which hosts are required for the malaria life cycle?
Humans/mammals (liver and blood stages, 11-14 days)
Anopheles mosquitoes (sexual reproduction, 7-14 days).
What is the cycle of plasmodium in Humans?
Sporozoites injected into the skin via infected mosq bite » reaches liver where sporozoites infect hepatocytes where they replicate and release merozoites into the bloodstream » infect RBCs and replicate further » leading to symptoms of malaria.
What occurs in the erythrocytic cycle (blood stage)?
Merozoites infect red blood cells (RBCs); clinical symptoms appear; ~5% become gametocytes.
How do gametocytes continue the malaria life cycle?
Migrate to skin and are taken up by mosquitoes during blood meals.
Life cycle of plasmodium in mosquito
The gametocytes in the mosquito gut undergo sexual reproduction:
» male gametocytes exflagellate, form ookinetes, which form oocysts.
» Oocysts produce thousands of sporozoites which tracel into mosquito salivary glands
How can sporozoites enter the human immune system after a mosquito bite?
Enter bloodstream or lymphatic system (to lymph nodes for antigen presentation).
What role do dendritic cells (DCs) play in the skin after infection?
Phagocytose sporozoites and present antigens to T cells.
How are exogenous(external) and endogenous(internal) antigens presented by DCs?
Exogenous - via MHC II to CD4+ T cells
Endogenous - via MHC I to CD8+ T cells
Cross-presentation also occurs - via porhyrin-2 to stimulate CD8+ T cells from exogenous sources.
What is the parasite's goal during early infection?
Evade immune recognition and rapidly reach liver for intracellular survival.
How do sporozoites infect hepatocytes?
Cross liver sinusoids and infect ~100 hepatocytes, sometimes migrating between cells.
What innate immune response is triggered in infected hepatocytes?
Release Type I interferons (IFN-α, IFN-β) to enhance antigen presentation.
Upregulate Interferon Alpha Receptors on neighboring hepatocytes.
Increase MHC Class I expression → enhance antigen presentation.
What is the adaptive response triggered in infected hepatocytes
Cytotoxic CD8+ cells recognise infected cells
Kill via:
By Fas/FasL-mediated apoptosis
Perforin/granzyme-mediated apoptosis.
What are the clinical issues that malaria can lead to?
Hepatomegaly (liver enlargement) by inflammation and cell debris clearance
Splenomegaly (Spleen enlargement) by filtering damaged RBCs, immune response extramedullary hematopoiesis
What is extramedullary hematopoiesis?
Production of RBCs outside the bone marrow
What are clinical symptoms during the blood stage of malaria?
Common - Malaise and fever.
Severe - Cerebral malaria, severe anaemia, respiratory distress.
What causes severe malarial anaemia (SMA)?
Loss of RBCs and reduced erythropoiesis.
What mechanisms contribute to RBC loss in malaria?
Direct destruction of infected RBCs, haemolysis of uninfected RBCs, phagocytosis of damaged RBCs.
How does bone marrow suppression affect malaria anaemia?
Decreased erythropoiesis despite high erythropoietin (EPO) levels.
What is the bystander effect in malaria anaemia?
For every infected RBC destroyed, about 12 uninfected RBCs are also lost.
What is haemozoin and its role in malaria?
Crystalline byproduct of hemoglobin digestion that activates inflammatory cytokine responses via TLR9 on macrophages.
toxic
Binds parasitic DNA
What dual role do macrophages play in malaria?
They remove damaged RBCs (beneficial) but excessive clearance of RBCs can worsen anaemia (pathogenic).
Summary table
Stage | Event | Host Response |
Skin | Sporozoite entry | DC uptake and antigen presentation |
Liver | Hepatocyte infection, replication | Type I IFN release, CTL response |
Blood | RBC infection → Fever, Anaemia | Antibody, Macrophage clearance, Bone marrow suppression |
Severe Disease | SMA, Cerebral Malaria, Respiratory distress | RBC loss, Hypoxia, Cytokine storm |
What causes dyserthropoiesis?
Disruption of RBC production
Cytokine release during infection inhibits erythropoisesis
Haemozoin impairs erythroid precursors and disrupts normal RBC maturation, contributing to anemia.
What does the spleen do in malaria?
Filters damaged or infected RBCs, and some production of RBCs (erythropoiesis)
Has a red pulp which removes RBCs, and a white pulp that has the adaptive immune response.
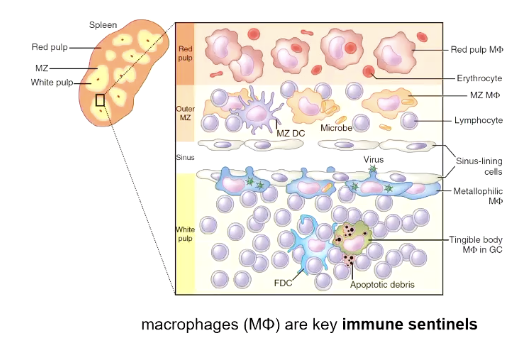
What do macrophages do in malaria?
Phagocytose infected RBCs
Produce oxidative bursts to kill parasites
Secrete cytokines = fever
enhance adaptive immunity
Why is malaria able to evade immune detection in RBCs?
Because RBCs lack MHC Class I, preventing antigen presentation.
What causes cyclical fever in malaria?
Synchronized rupture of infected RBCs rereleasing PAMPs and DAMPs that trigger a cytokine storm
Induces fever via COX-2 and prostaglandin production
What are the main antibody functions in malaria immunity?
Neutralization, opsonization, complement activation, and transmission blocking.
What do T cells do in malaria?
Tregs produce IL-10 to dampen immune response and reduce inflammation
Thelpers produce IFN-gamma to activate macrophages to aid B cells
Why is malaria immunity slow to develop?
Immunity is species- and stage-specific and requires repeated exposure; lost without ongoing exposure.
How does antigenic variation help malaria parasites evade immunity?
By varying proteins like AMA-1 and PfEMP1 to avoid antibody recognition.
What role does PfEMP1 play in severe malaria?
Binds endothelial receptors causing vascular occlusion and cerebral malaria.
What is the significance of rosetting in malaria?
Clumping of infected and uninfected RBCs, contributing to disease severity.
What is the difference between clinical and sterile immunity in malaria?
Clinical immunity controls symptoms but parasites can persist; sterile immunity completely clears infection.
What challenges does malaria antigen diversity pose for vaccine development?
It requires broad coverage against many variants and life stages.