CHEM 1112: Nuclear Chemistry
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Nuclear Composition
nuclei contain two types of nucleons:
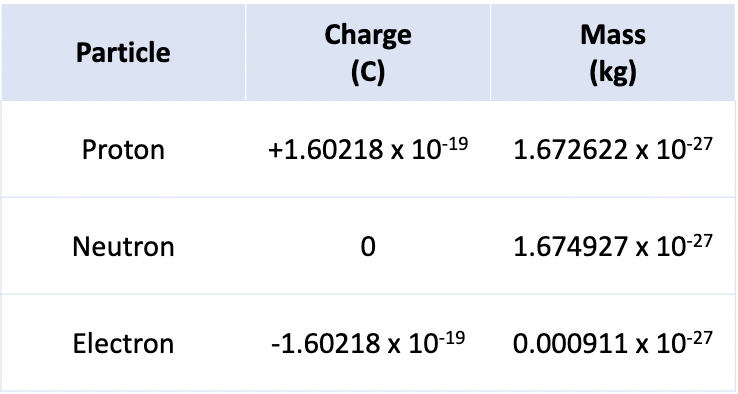
protons (+ charge)
neutrons (neutral charge)
Proton, Neutron, and Electron charge/mass
Atomic # vs. Mass #
Atomic #:
Z
number of protons in nucleus
Mass #:
A
number of protons + neutrons in nucleus
Principal Isotropes of H
protium
most abundant
deuterium
tritium
Nuclide
a particular nucleus
characterized by # of protons (Z) and neutrons (N) that they possess
chlorine-37 is a nuclide
Isotopes
atoms of the same element whose nuclei contain different numbers of neutrons
chlorine-37 is an isotope of chlorine
some isotopes are unstable, and tend to rearrange or disintegrate
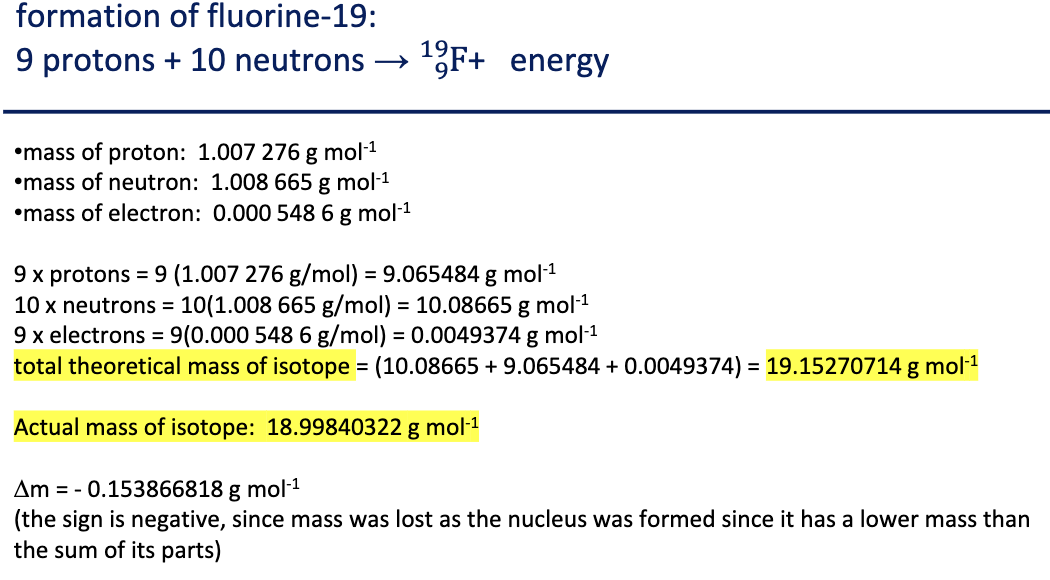
Mass Deficit
∆m
a nucleus has a lower mass than the sum of its parts
this is because so much energy is released upon the formation of a stable nucleus, that the nucleus actually loses mass
can be calculated by comparing the “sum of the parts” to the observed mass
Nuclear Binding Energy
a positive mass defect (∆m) times the speed of light (s) to find a change in energy (∆E)
energy corresponding to the mass defecit
positive sign represents the energy required to disassemble the nucleus
nuclides with mass # around 56 are the most stable
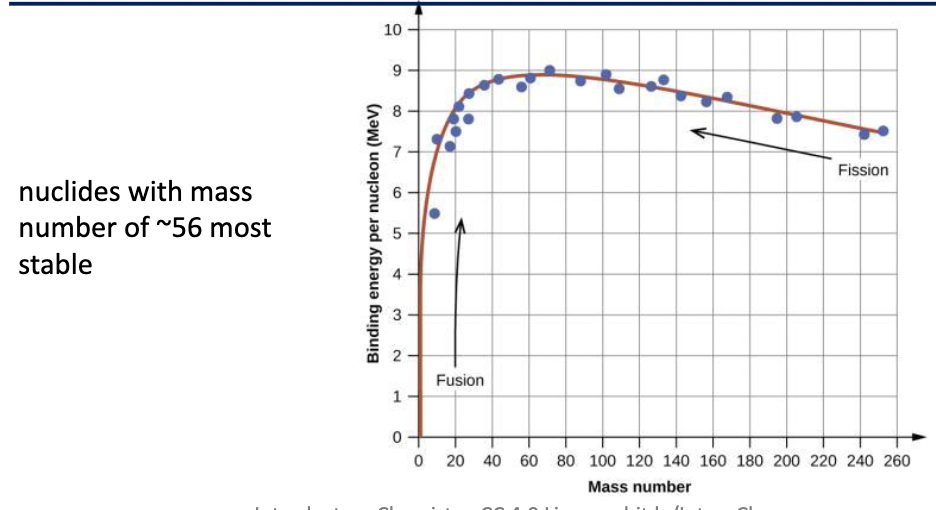
Relative Nuclear Stability
to compare which nucleus of two separate nuclides is more stable, we must compare binding energy per nucleon
∆E/nucleon
higher binding energy per nucleon → more stable
Unstable Nuclei
too many or too few neutrons (for the # of protons) which causes the nucleus to be unstable
unstable nuclei may become more stable by giving off this excess matter and/or energy, or by capturing a nearby electron
nuclei that are too large (too many protons in the small area of the nucleus) will be unstable
such nuclei may become more stable by giving off the excess matter or even splitting to form two new nuclei
all isotopes of elements with atomic numbers greater than 83 are unstable
Unstable nuclides decay to form more stable nuclides
Belt of Stability
unstable nuclides in green
stable nuclides in blue
generally, most stable isotopes have n > Z
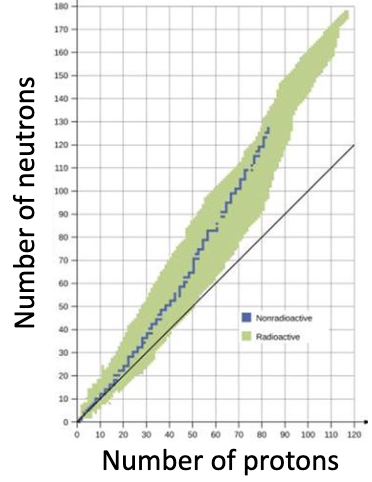
Nuclear Stability
balance between attractive forces and repulsive forces
attractive forces only over very very short distances
elements with large atomic # (Z) need more neutrons
this increases the value of net nuclear binding force
this increases the distances between protons
this decreases repulsion
more stable nuclides have roughly equal numbers of neutrons and protons
Nuclear Decay
decomposition of unstable nuclides into more stable ones, often accompanied by a change in mass
radioactive decay
decay MUST conserve mass # and charge !!!!!
Radioactivity
spontaneous nuclear disintegration of atoms with emission of particles or electromagnetic radiation.
radiation that is emitted from a radioactive isotope may include:
alpha particles
beta particles
positrons
gamma rays
Alpha Particle
ɑ
high energy helium nuclei consisting of two protons and two neutrons
mass # is 4
charge is 2+

Beta Particle
β
high energy negatively charged particle (electron)
mass # is 0
charge is -1
will not exist long, usually will promptly collide with an electron
both will be annihilated with the release of energy (usually 2 gamma photons)
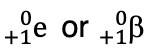
Positron
composed of + charged particles (positrons)
mass # is 0
charge is +1
will not exist long, will promptly collide with an electron
both will be annihilated with the release of energy (usually 2 gamma photons)
Gamma Photons
𝛾
very high energy electromagnetic radiation
mass # is 0
charge is 0
a photon
very penetrating, and can pass through a human body
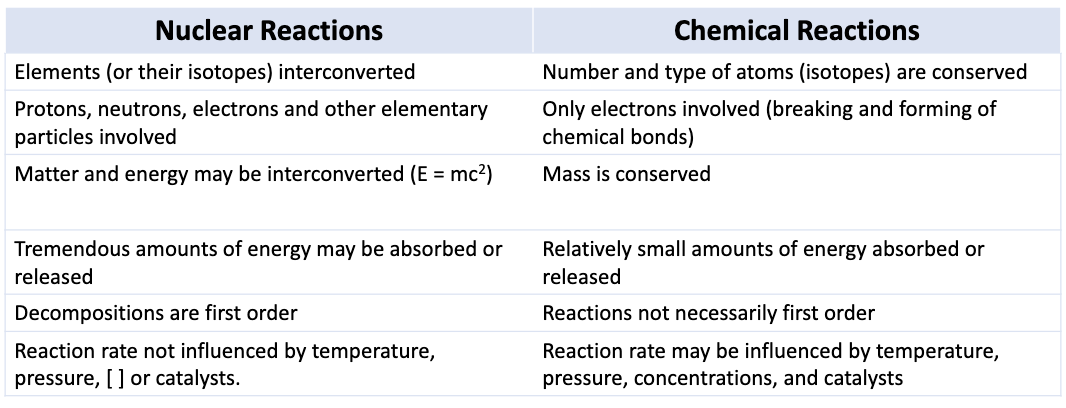
Chemical Change
in chemical change, no material is created or destroyed
end up with the same number/types of atoms
net charge is conserved
energy may be absorbed or produced, but any mass change is not measurable
Radioactive Decay
total mass number is conserved
total charge is conserved
types of atoms will usually change
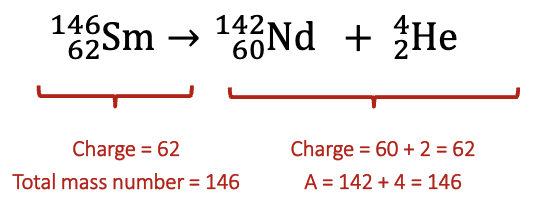
Nuclear vs. Chemical Reaction
ɑ-emission
Z decreased by two units and A decreases by 4 units
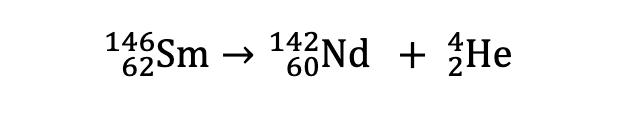
β-emission
Z increases by one unit and A does not change
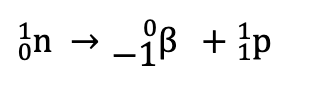
Positron Emission
Z does not change, and A is decreased by 1 unit
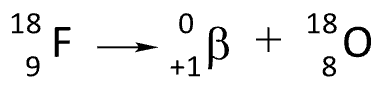
Electron Capture
Z does not change, and A is decreased by 1 unit
an inner shell electron of the atom is a reactant
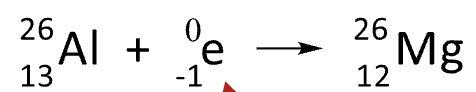
Types of Nuclear Decay
Too big ?
ɑ-emission
Protons too large ?
β-emission
Protons too small ?
positron emission
electron capture
Decay Sequences
series of radioactive decays that an unstable nucleus undergoes in order to become stable
may either be ɑ-decay or β-decay
all decays obey the 1st order rate law
Rate= k[A]
t1/2 = 0.693/k
ln[A]t / [A]o = -kt
Unstable Nuclei
stable isotopes remain stable, whereas unstable isotopes continuously disintegrate
thus, every element should be composed of ONLY stable isotopes
unstable isotopes present either have half-lives longer than the age of the earth, or are products of the decay of these nuclides
Nuclear Transmutation
transformation of one element into another
occurs during nuclear reactions
is not restricted to natural decay processes
can also occur through induced nuclear reactions
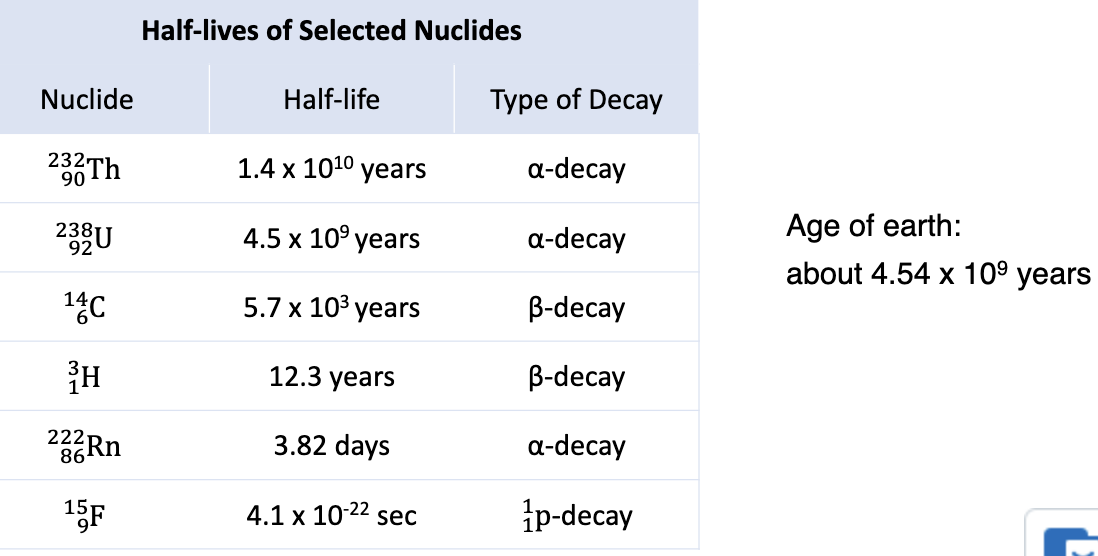
Induced Nuclear Reaction
occurs when a nuclear projectile collides and reacts with another nucleus
classified according to the nature of the nuclear projectile
most common: neutron-capture
Neutron Capture
always exothermic
product is a metastable, highly excited nuclide that usually decays by either proton or gamma emission
neutrons could come from the sun, or produced in a nuclear reactor
the earths atmosphere is constantly bombarded with solar neutrons
can be captured by the most abundant element in atmosphere 14N to form unstable 15mN, which will fragment into 12C + 3H
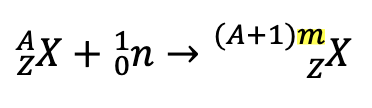
Fission
when heavy nuclides fragment
Fusion
when light nuclides combine
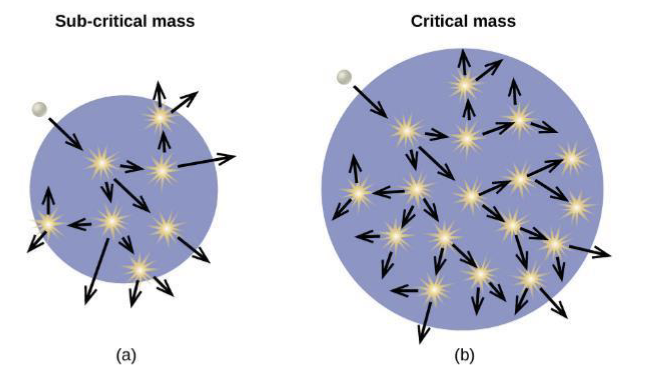
Fission Chain Reaction
the fission of a large nucleus produces two or three neutrons, each of which is capable of causing fission of another nucleus by the reactions shown attached.
if this process continues, a nuclear chain reaction occurs
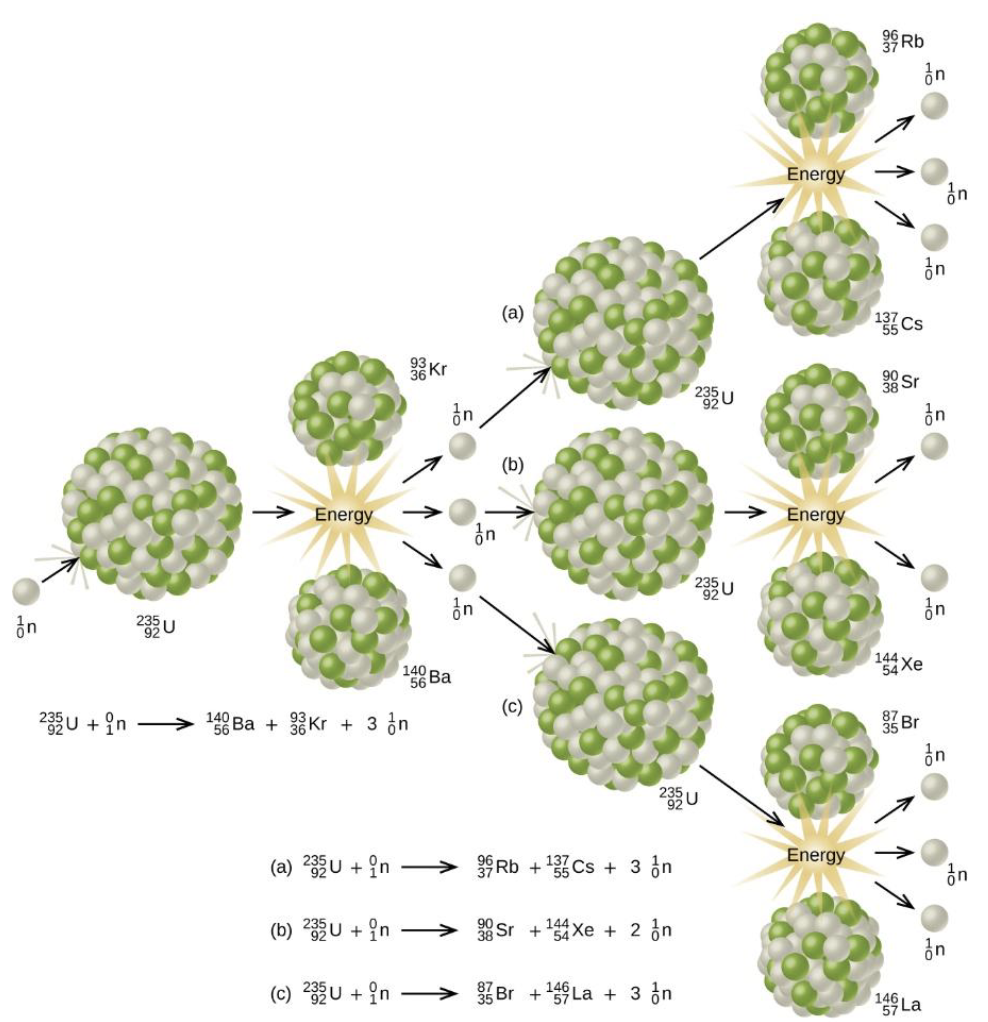
Subcritical Mass
fissile material is too small and allows too many neutrons to escape the material, so a chain reaction does not occur
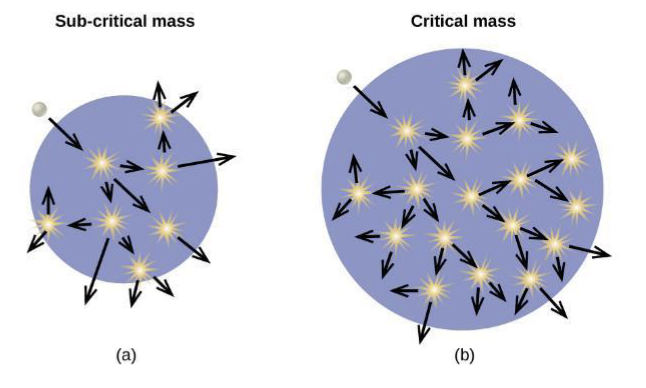
Critical Mass
a large enough number of neutrons in the fissile material induce fission to create a chain reaction
Advantages of Nuclear Fission (energy production)
generates a tremendous amount of electricity from a small amount of fuel
generates no air pollution, or greenhouse gases
Problems with Nuclear Fission (energy production)
potential for nuclear accidents as the fission reaction can overheat
the disposal of products brings issues, as they may be radioactive and have long half-lives
Advantages with Nuclear Fusion (energy production)
generates a tremendous amount of electricity from a small amount of fuel
generates no air pollution, or greenhouse gases
provides about 10x more energy per gram of fuel than fission
does not produce radioactive waste products
Problems with Nuclear Fusion (energy production)
very high temperatures required for fusion to occur, and no existing material can withstand those temperatures for long
need to contain using magnetic fields
Tokamak Fusion Reactor
uses a powerful magnetic field to confine plasma
able to reach temperatures around 100-150 million oC
difficult to attain conditions to allow the reaction to be self-sustaining and controlled
record is 22 minutes of maintained plasma reaction, reached a few months ago
Applications of Radioactivity
radiocarbon dating
applications to human health
diagnosis of disease
treatment of disease
Radiocarbon Dating
in the upper atmosphere 14C both forms and degrades at a constant rate, thus there is a relatively constant ratio of 14C:12C in the atmosphere
from this, scientists can work backwards to determine the length of time since an organism had ‘died’
can date organisms up to 50,000 years old with 5% accuracy
Uranium/Lead Dating
can estimate age of older objects that were never alive, using other techniques
U-238 decays to Pb-206 with a half-life of 4.5×109 years
Positron Emission Tomography
PET scan
detects gamma rays emitted during positron annihilation
used commonly to detect areas of high metabolic activity
active areas of brain
sites of tumours
Warburg Effect
the rate of glycolysis is elevated in almost all tumours
useful for potential treatment strategies
useful for diagnosis of cancer
Glycolysis
set of reactions that converts glucose to pyruvate or lactate
Boron Neutron Capture Theory
novel approach where tumour cells are allowed to pick up compounds rich in boron-10.
the tissues are then bombarded with neutrons
boron-10 will capture the neutrons, forming radioactive boron-11
targets:
brain tumours
head and neck cancer
melanoma
liver cancer
lung cancer
mesothelial tumour
breast cancer
able to target tumour cells since target cells are actively producing proteins that contain tyrosine