Photosyntheis and the Oxygen Electrode
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
How does the oxygen electrode work
O2 generated by photosysnthesi
treavels to a platinum electrode
undergoes a reduction reaction
generates H2O2
Causes a current to be generated
Proportional to the amount of O2 in the system
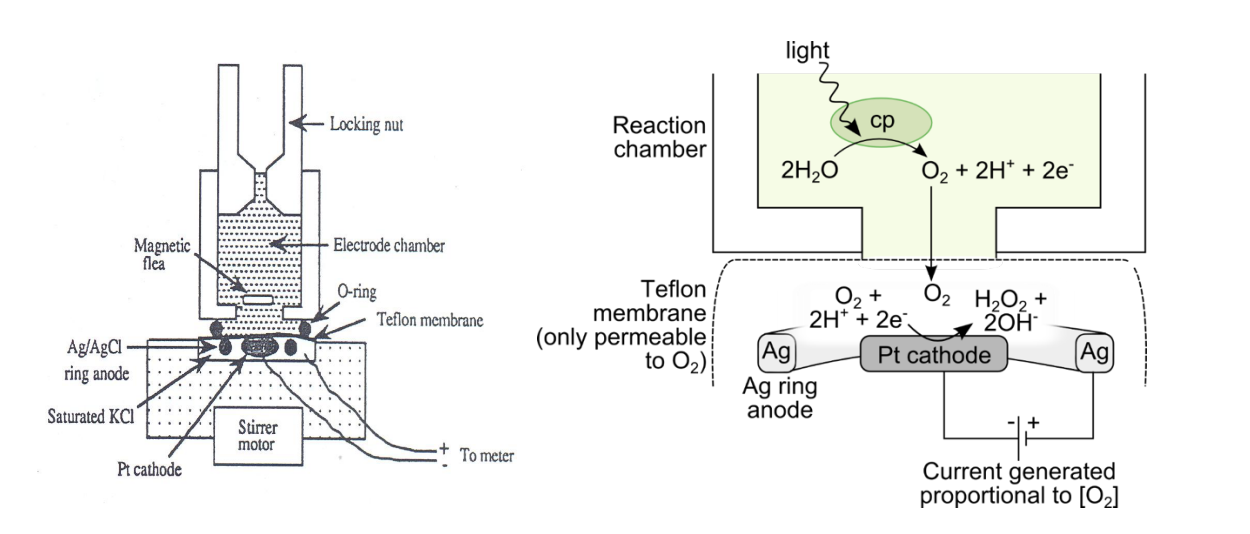
For oxygen to be generated
must be a flow of electrons through the photosynthetic pathway
electron acceptors and donors must be present!
→
It is possible to substriute with artificial electron acceptors and donors
to measure the in vitro activity of photosynthetic machinery
Examples of artifical e- acceptors
Ferricyanide→ only accept electrons from PSI
Pheylquinone→ only accepts electrons from plastoguinone of PSII
not from PSI !
Preparation of lettuce chloroplasts
remove central veins
chilled mortar and homegice with ice cold sucrose medium
pour over a wet muslin
and squeeze through 4 layers into beaker
immediately into centrifuge tube
centrifuge for 3 mins
chloroplasts with sediment→ pellet isn’t pure but is good enough
pour off supernantent
add more sucrose medium and sqirl to suspend pellet
Why such a high sucrose concentration in the buffer?
This is the approximate concentration of solutes in the chloroplasts, making this an isotonic buffer.
This means the osmotic potential of the buffer matches that of the biological sample,
so there is no danger of osmolysis of the organelles.
Measuring chlorophyll content
acetone and chloroplasts into centrifuge tube
cetifuge
zero spectrometer and put in
calculate amount of chlrophyll in chloroplast smaple→ can use beer’s law but limitations!
replace chlroplasts on ice in the dark
Why kept on ice?
When you grind up the tissue you will disrupt the lysosomes and vacuoles.
This will release protease enzymes which will degrade your sample, therefore keeping the extracts on ice will reduce the activity of these enzymes and protect your sample.
Why use acetone to extract chlorophyll?
Chlorophyll is a lipid-soluble molecule,
and is found bound to chlorophyll-binding proteins in the thylakoid membrane
Acetone dissolves the membrane and releases the chlorophyll.
Why can’t really use Beer’s law
got a micture of chlorophyll a and b which has different absorption peak range thingy
Calibrating the oxygen electrodes
few crystals of sodium dithionite→ reducing agent
will remove all dissolved O2
do not put lid on
reading should fall to 0 in 30 seconds
wash reaction vessel with distilled water to remove sodium dithionite
shake RO water to aerate and saturate with oxygen
press + to get reading close to 1000 mV
Reading can now be converted to O2 concentration
since solubility of O2 in air-saturated water is 0.255 micromol O2ml-1
and the volume you are placing is 1.5mml
Determining if herbicides affect photosynthesis
Remove any liquid in current chamber
Add to O2 electrode
buffer (with KCN)
ferricyanice
Add N2 into liquid until reading is 200mV
allow to stabilise
add chloroplasts to liquid under the surface of the liquid
put the lid on
cover with a black pastic bag
start timer and take readings every 20 seonds
repeat more without plastic bag
Remove and add herbicide
Take readings again
Why use KCN in the buffer?
KCN is toxic because it is an inhibitor of cytochrome c oxidase, which is one of the components of the respiratory electron chain.
Use to inhibit mitcohondria respiration so only measure oxygen related to photosynthesis
Determining the target of the herbicide
repeat again but add phylquinone too
Also add the herbicide
Only accepts electrons from PSII
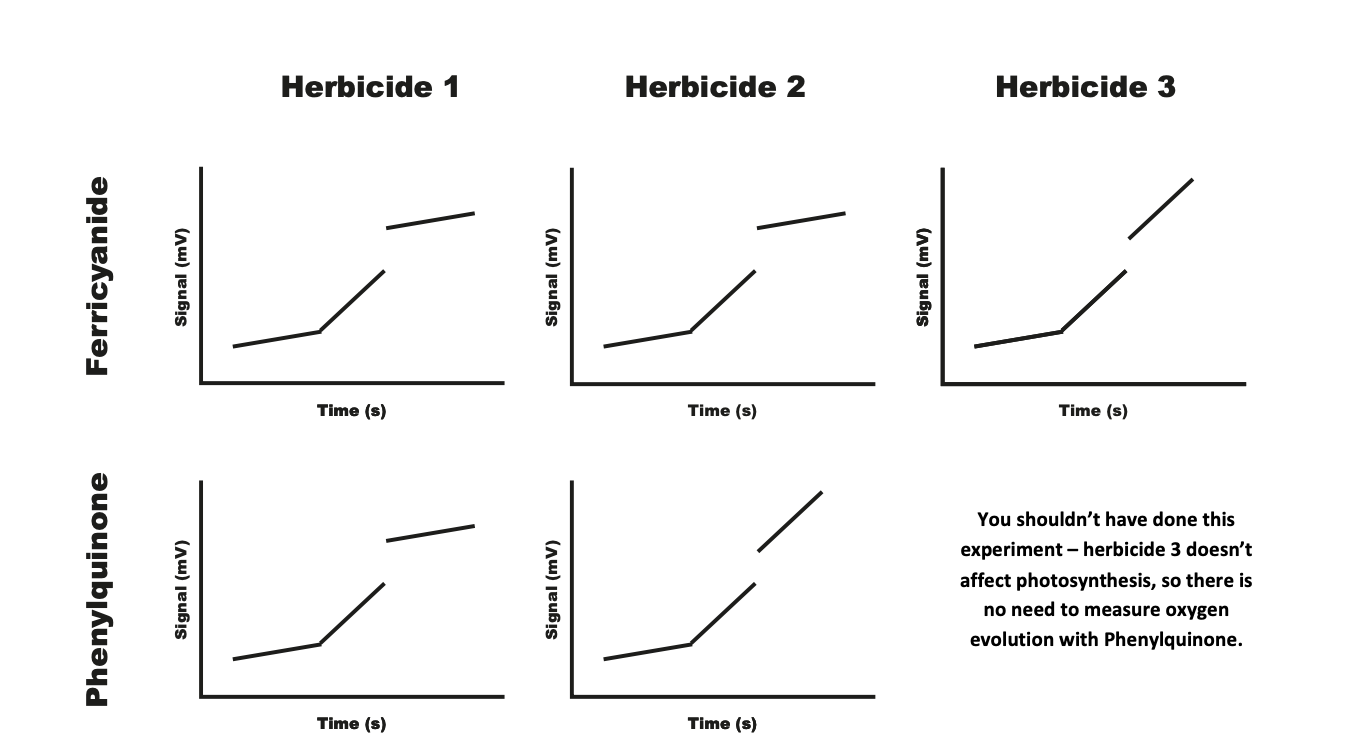
Results
Herbicide 1: must target PSII becaue when PhQ is used→ oxygen evolution is inhibited
Herbicide 2: reduction with ferricyanide but no reduction with PhQ→ does not target PSII→ do not know if it targets PSI
Herbicide 3: no reeduction in O2 with ferricyanide→ herbicide does not target photosynthesis e.g linezoid 1 inhibits protein translation in the chlroplast but not in nuclei
Plot the electrode readings from Table 1 against time on the graph paper provided.
By this means you can obtain a value for the rate of photosynthetic oxygen evolution, expressed as µmolO2 min-1 mgChl-1 .
You will need to include the following pieces of information: - The solubility of O2 in air-saturated water is 0.255 µmol O2 ml-1 at 20o C - The concentration of chlorophyll in your chloroplast suspension, after any volume adjustments you may have made - Remember that photosynthesis doesn’t occur in the dark!
Photosynthetic rate= rate in ligh- rate in dark Substrating background rates!)
Covert from mV→ O2 concnetration
e.g 1000mV = 0.255, so 1mV =2.55 ×10-4
O2 concentraion→ O2 amount
Determne amount of chlorophyl in the chamber chl c→ amount
Divide by amount of chlorophyll
In the practical we will use a buffer with the following composition to extract chloroplasts: 0.38M sucrose, 0.07 M K2HPO4, 0.01 M KCl, adjusted to pH 7.5. Why?
It maintains an appropriate physiological pH
It resembles the high concentration of sucrose in the normal cytosol
It provides an isotonic solution which prevents the chloroplasts from bursting.
Which part of the molecule is responsible for the molecule being able to absorb light directly?
Aromatic porphyrin ring
Which processes take place in the stroma of a chloroplast?
NADPH production
Calvin cycle
ATP production
What type of proteins are chlorophyll molecules associated with in the cell?
Integral membrane proteins in the thylakoid membrane
Artifical electron transort chain acceptors
Phenl quinone
Ferricyanide
Source of electrons for plastoquinone
PSII
Source of electrons for Ferredoxin
PSI
Source of electrons for Pheylquinone
PSII
Source of electrons for ferricyanide
PSII and PSI
Source of electrons for Plastocyanin
Cytochrome bgf
Role of sodium dithionite
reducing agent
Role of H2O
polar electron donor
Plastoquinone
lipid soluble hydrogen carrier
Plastocyanin
electron carrying protein including copper
NADP+
Polar electron acceptor
IF PSII is stopped, what parts of photophosphylation are stopped
O2 prodution
NADPH production
Linear electron flow
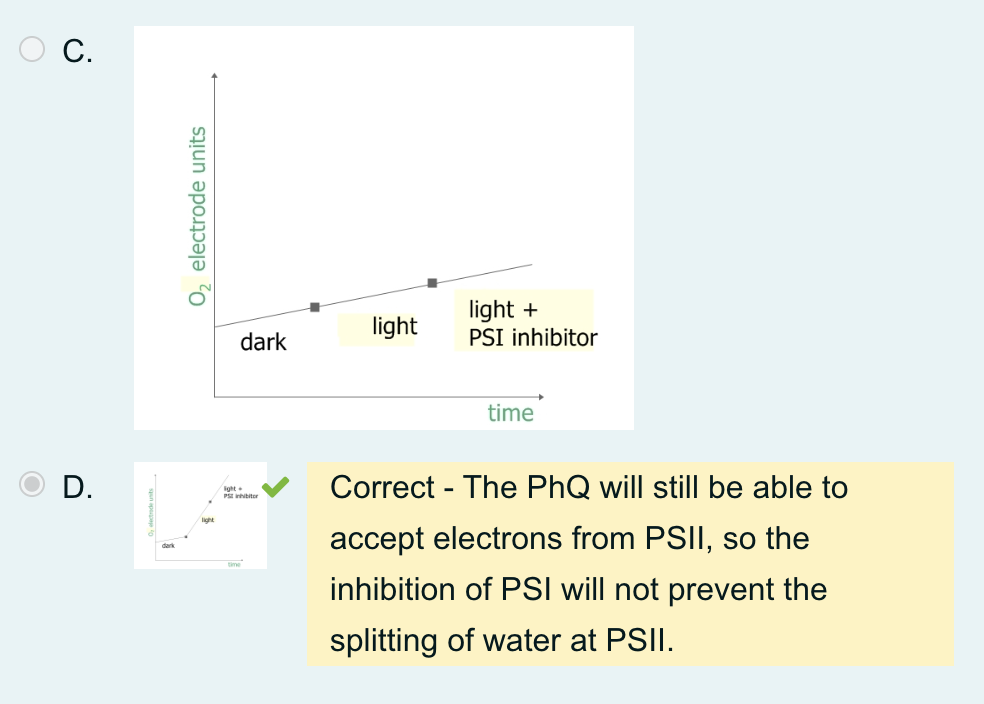
A student set up a chloroplast extraction in an appropriate buffer within an oxygen electrode, as in the week 7 experiment.
She added PhQ as an electron acceptor and paraquat, a PSI inhibitor, to her chloroplast preparation after a short time and measured the oxygen electrode reading at regular intervals.
Which graph below best reflects her results?
A student sets up the oxygen electrode to measure photosynthesis using the instructions in the practical schedule. When they start recording, there is a large decrease in the O2 concentration over time when the chloroplasts are in the dark. What is the most likely mistake they have made in setting up the electrode?
They forgot to add KCN
without KCN present there will still be respiration occurring which will consume the oxygen present
Best buffer to use
The buffer should have a pH of between 7 and 7.5 to match cytosolic pH,
and the 0.38 M sucrose concentration makes the buffer isotonic to the chloroplasts to help maintain their structure.
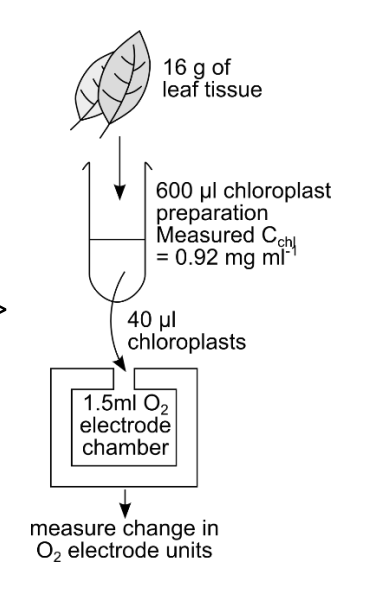
An oxygen electrode was calibrated with sodium dithionite to define 0 mV, and air saturated water to define 1000 mV. Chloroplasts were prepared as per the IA cells practical, then 40 µL of chloroplasts were added to an electrode chamber containing 1410 μL phosphate buffer, 40 μL ferricyanide and 10 μL KCN. The following data were recorded:
Lightrate-dark rate
Light→ 558-42
Dark→ 359-352
Rate= 130mVmin-1
convert mV to O2 using the solubility of oxygen
convert o O2
0.03315
Convert concentraion to amount
n=cxv because we have 1.5ml
n= 0.03315 ×1.5 = 0.0497
An experiment was set up as follows:
An oxygen electrode was calibrated such that 1000 mV corresponds to fully air-saturated water.
6 g of rat liver was homogenised, and 3 mL of mitochondria were extracted.
20 μL of mitochondria were added to 2.98 mL of an ADP containing buffer in an oxygen electrode chamber.
Given that a decrease of 133 mV was recorded over 60 seconds, and assuming that the solubility of O2 in water under the experimental conditions is 0.255 μmole O2 mL-1, calculate the rate of respiration as μmole O2 min-1 gliver-1.
mV → O2 concentration
Concentration→ amount in chamber
Amount in chamber→ amount in total extact
divide by g of liver in tissue