IB chemistry R3.4
1/51
Earn XP
Description and Tags
1-2,3,4-5,9-10,11-12,13
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What is a nucleophile
An electron-rich species that is attracted to regions of positive charge it is a reactant that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons.
what happens in a nucleophilic substitution reaction
a nucleophile donates an electron pair to form a new bond, as another bond breaks producing a leaving group
how does a Nucleophilic substitution reaction look
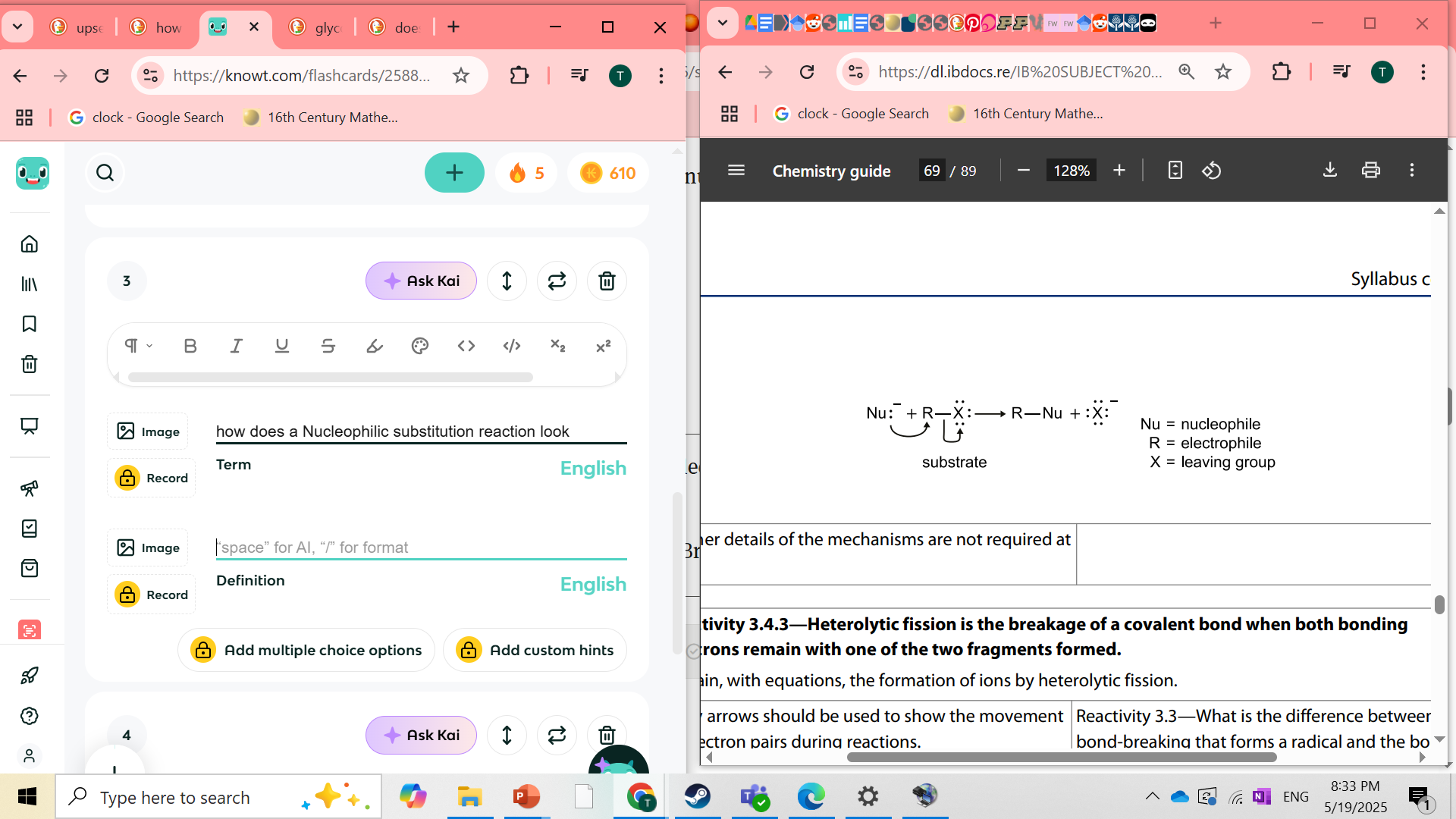
what is heterolytic fission
the breakage of a covalent bond when both bonding electrons remain with one of the two fragments formed
what is an electrophile
an electron poor species that accepts a pair of e- from the nucleophile
what do curly arrows show
the movement of e-
what is the most important thing an electrophile hould have
a low-energy unfilled orbital
what usually doens’t leave during a nucleophilic substitution reaction
an H- ion as it is unstable
what are 3 things that happen in a nucleophilic substitution reaction
a nucleophile donates a pair of electrons to form a new bond
a bond in the electrophile breaks to produce a leaving group
the nucleophile is substituted in place of the leaving group.
where do electrons go in heterolytic fission
to the atom with higher electronegativity
why can alkenes undergo electrophilic addition reactions
the pi bond is relatively weak and reactive because it is on the outside and so there is a high e- density around the double bond
what can be used as a chemical test for an alkene
Bromine water changes from brown to colorless
which Hydrogen Halides react with alkenes in order from least to most reactive and why
HI<HCl<HBr because the bond is getting longer and a longer bond, a weaker one, so faster to break
how can water react with an alkene
it can’t alone, so it needs H+ ions in order to form enough H3O+ molecules which
when is something an electrophile
if it is taking the electrons of an atom or bond
what are the only 2 ways that a C compound with 4 bonds can undergo a nucleophilic substitution reaction
SN1 or SN2 mechanism
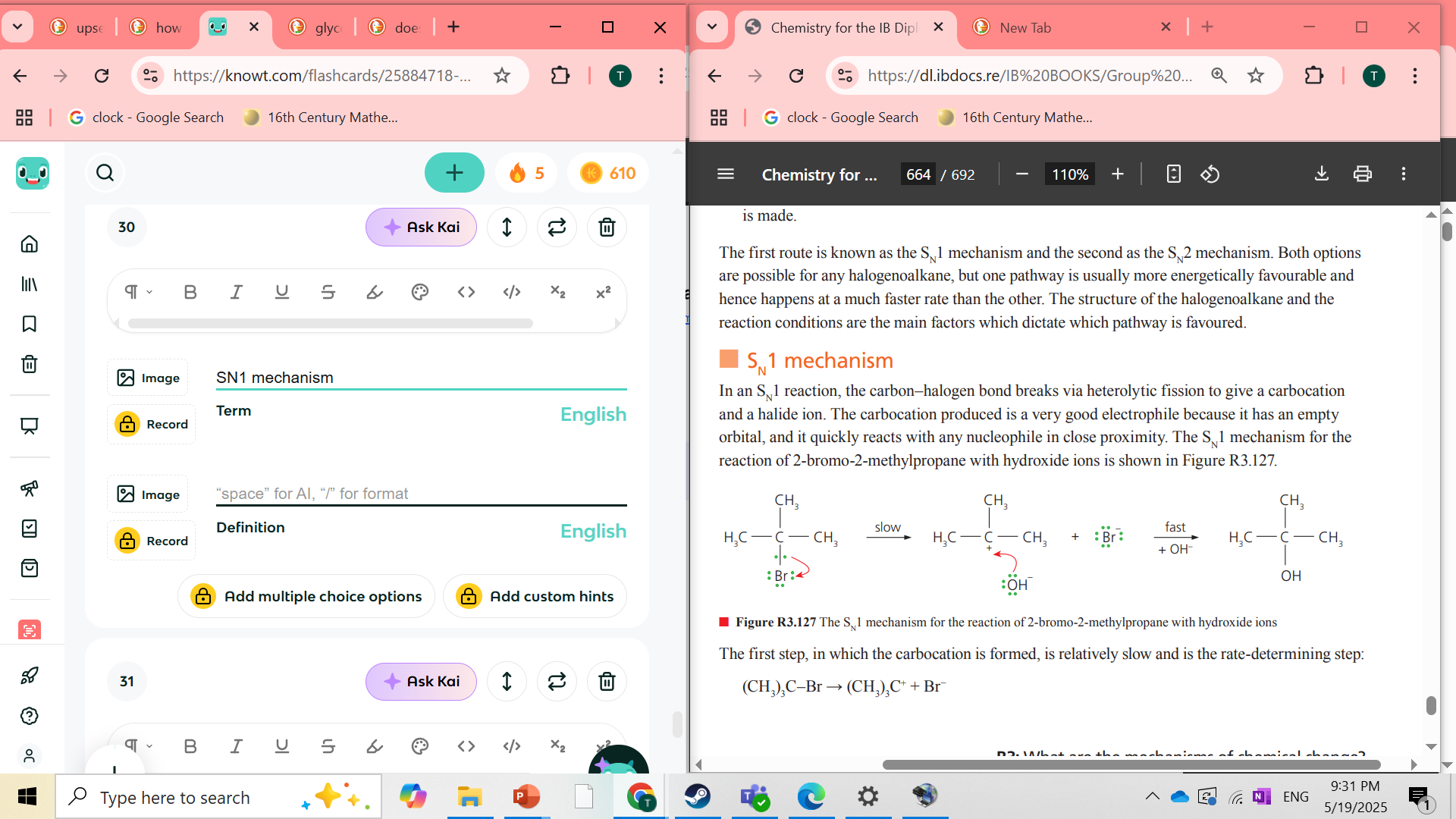
what is SN1 mechanism step
first the Carbon Halogen bond breaks via heterolytic fission and gives a carbocation and a halide ion. the carbocation has an empty orbital and is ready to bind to any nucleophile
SNA mechanism is ——molecular
uni
how is the rate of reaction of SN1 reaction calculated and what order is it
Rate=k x concentration of halogenoalkane
in SN1, rate is the order of — meaning
1, it depends on 1 concentration
why do we not measure the rate of reaction in SN1 of both steps
rate depends on the slower elementary step, the first one
what is a reaction mechanism
it is a reaction that occurs in multiple steps and each one is called an elementary step
what is an intermediate
a substance that is formed in one elementary step and used up in the next one
what is a transition state/ activation complex
a substance that is temporary and unstable where old bonds break and new ones form and it is represented by a dashed line
what is activation energy
it is the energy needed to make the activation complex/ transition state
what conditions to SN1 reactions require
a weak nucleophile
a protic solvent (can form HB)(the HHB act as a cage and help the nucleophile)
tertiary haloalkane because it has high steric hinderence
what does SN2 mechanis require
a strong nucleophile
an aprotic solution (doesn’t form HB)
primary haloalkane because it doesn’t have high steric hinderence
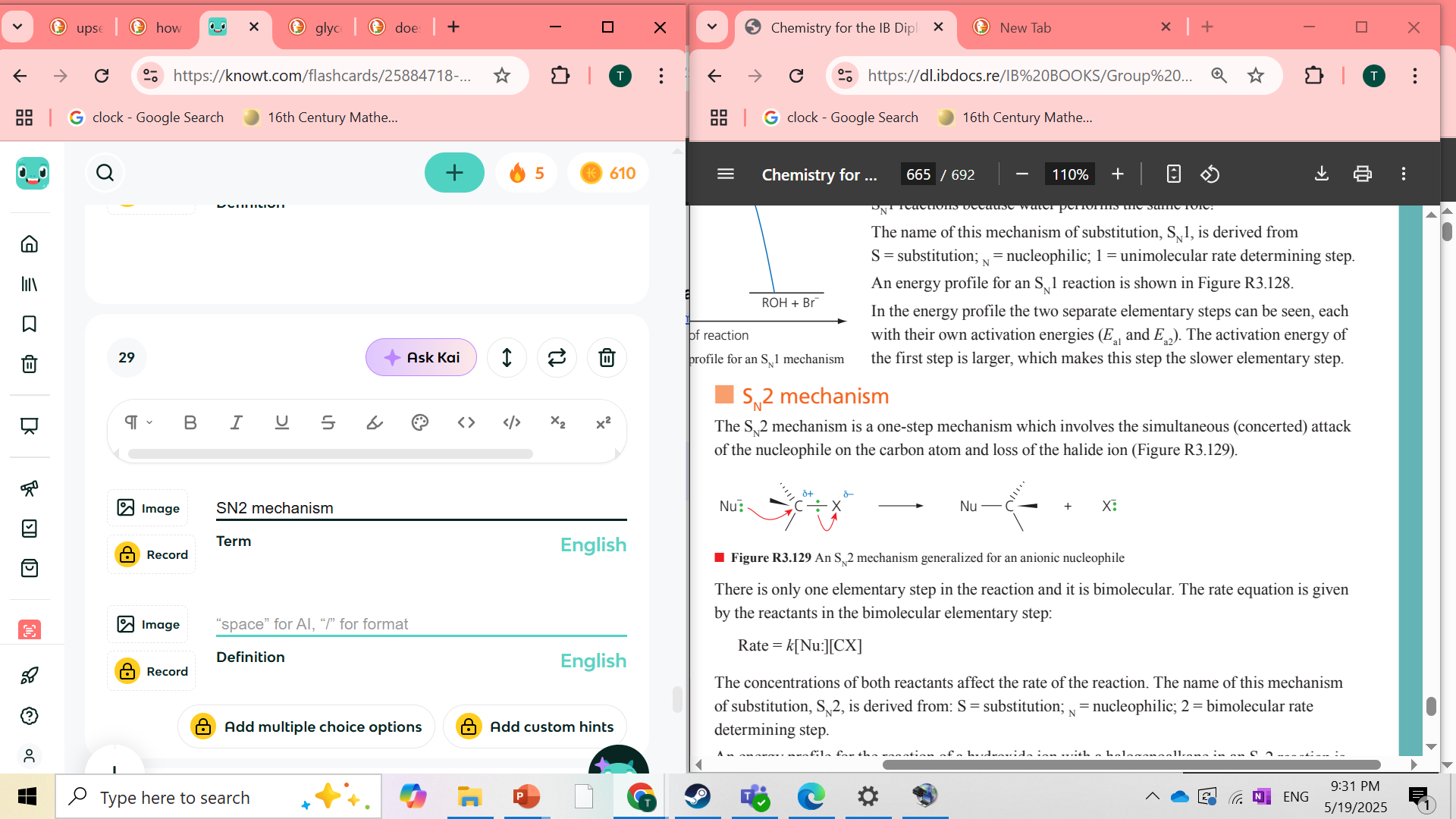
what is SN2 mechanism
it is a mechanism that involves the simultaneous attack of the nucleophile on the Carbon atom and loss of the halide ion. the step is bimolecular
SN2 mechanism
SN1 mechanism
what are the reactants and products of halogenation
alkene+X2-dihaloalkane
what are the reactants and products of hydrohalogenation
alkene+HX-haloalkane
what are the reactants and products of hydration
alkene+HOH-alcohol (Wich con. H2SO4)
what are the reactants and products of hydrogenation
alkene+H2-alkane with Pt or Ni catalyst
what is steric hinderance
bulkiness
SN1 is —--specific
non-stereo
which mechanism do secondary haloalkanes do
both SN1 and SN2
how is rate affected by the identity of the leaving group
the lower the bond enthalapy and the Electronegativity of the leaving group, the easier it is to remove I>Br>Cl
how does the energy profile of SN2 mechanism look
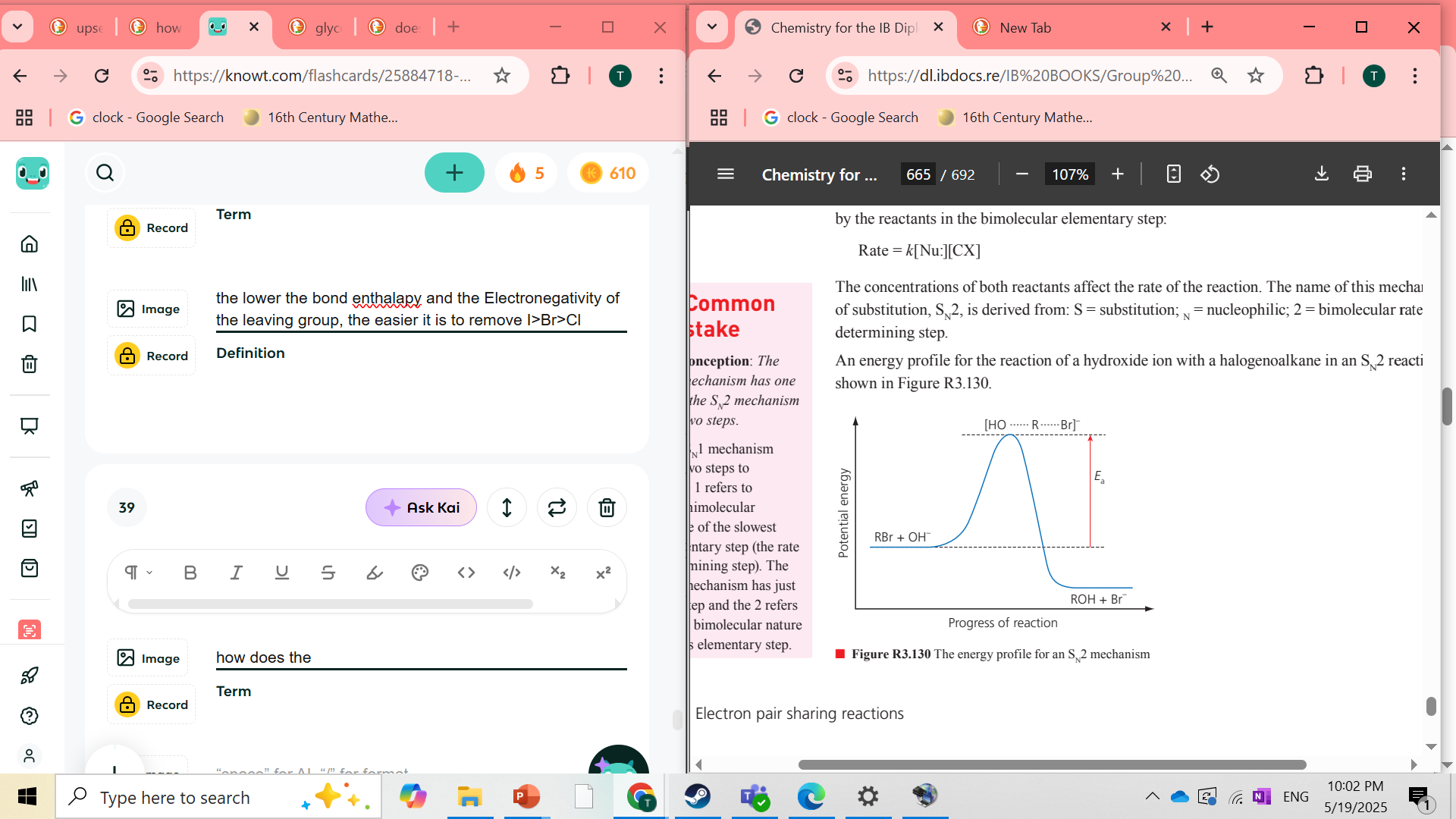
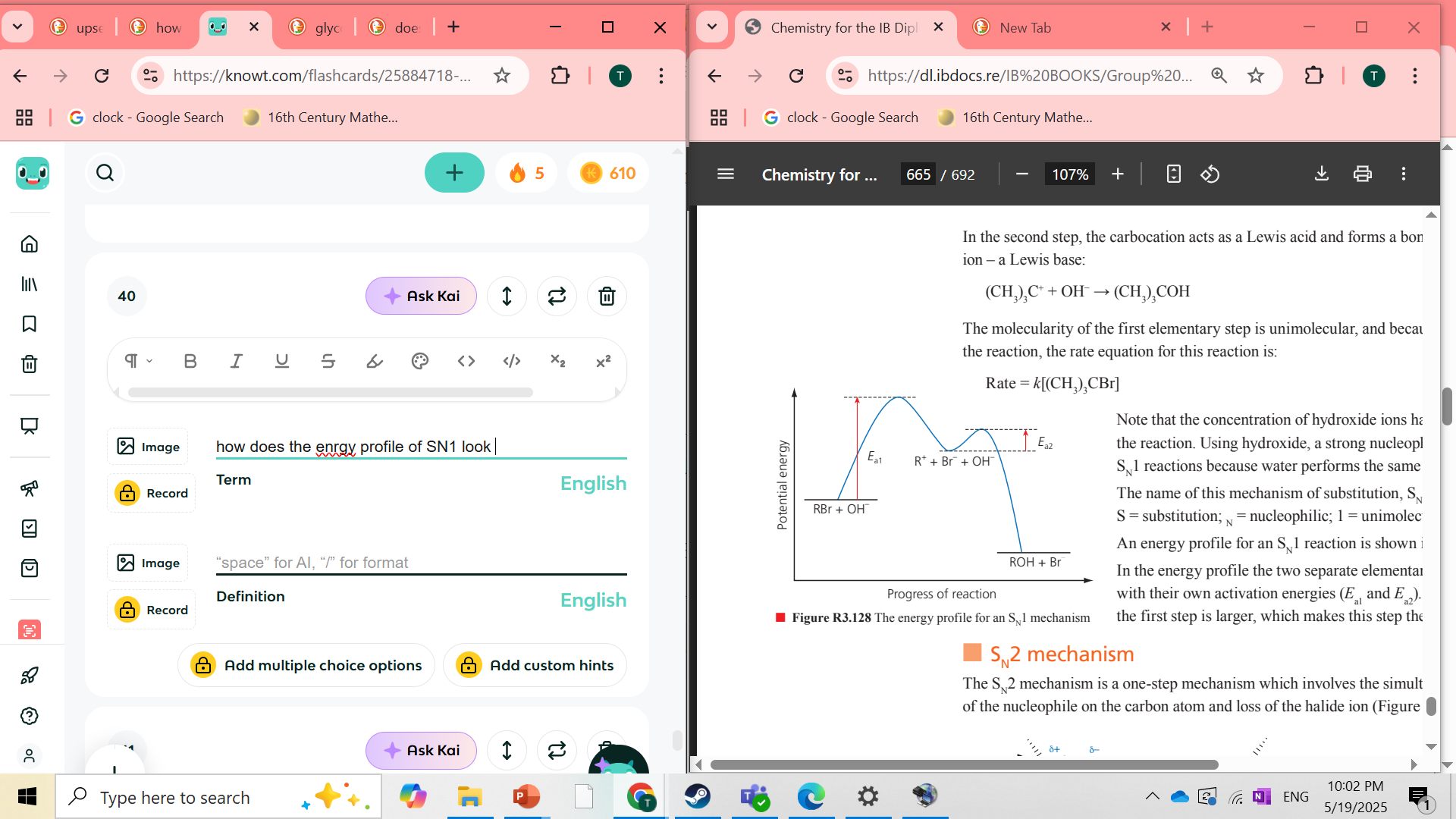
how does the enrgy profile of SN1 look
order the carbocations from least to most stable
1<2<3
in a symmetrical alkene, which electrophilic addition reactions would result in the same product
all of them because switching the location of the atoms will result in the same product
in an asymmetrical alkene, which electrophilic addition reactions would result in the same product
with a Hydrogen Halide, there are 2 products
with an X2, 1 product
with HOH, 2 products
if there were two products that could come out of an electrophillic addition reaction with an asymetrical compound, which would be favored
the one with the more stable carbocation, so 3>2>1
why are tertiary carbocations more stable than primary
there are 2 theories here is the one that makes sense: hyperconjugation, which is a concept in organic chemistry where electrons from a sigma bond (like C-H) are shared with an adjacent empty or partially filled orbital, which helps stabilize the molecule.
what does an SN1 mechanism form
racemic mixture, 50% D, 50%L
what is Markovinkov’s rule?
if there is HX, put the H where there is more H, rich get richer
why is benzene not a strong nucleophile despite having pi orbitals
it is reluctant to give away the six delocalized pi electrons and lose the stability that arises from extensive delocalization
how does an electrophilic substitution reaction with benzene look
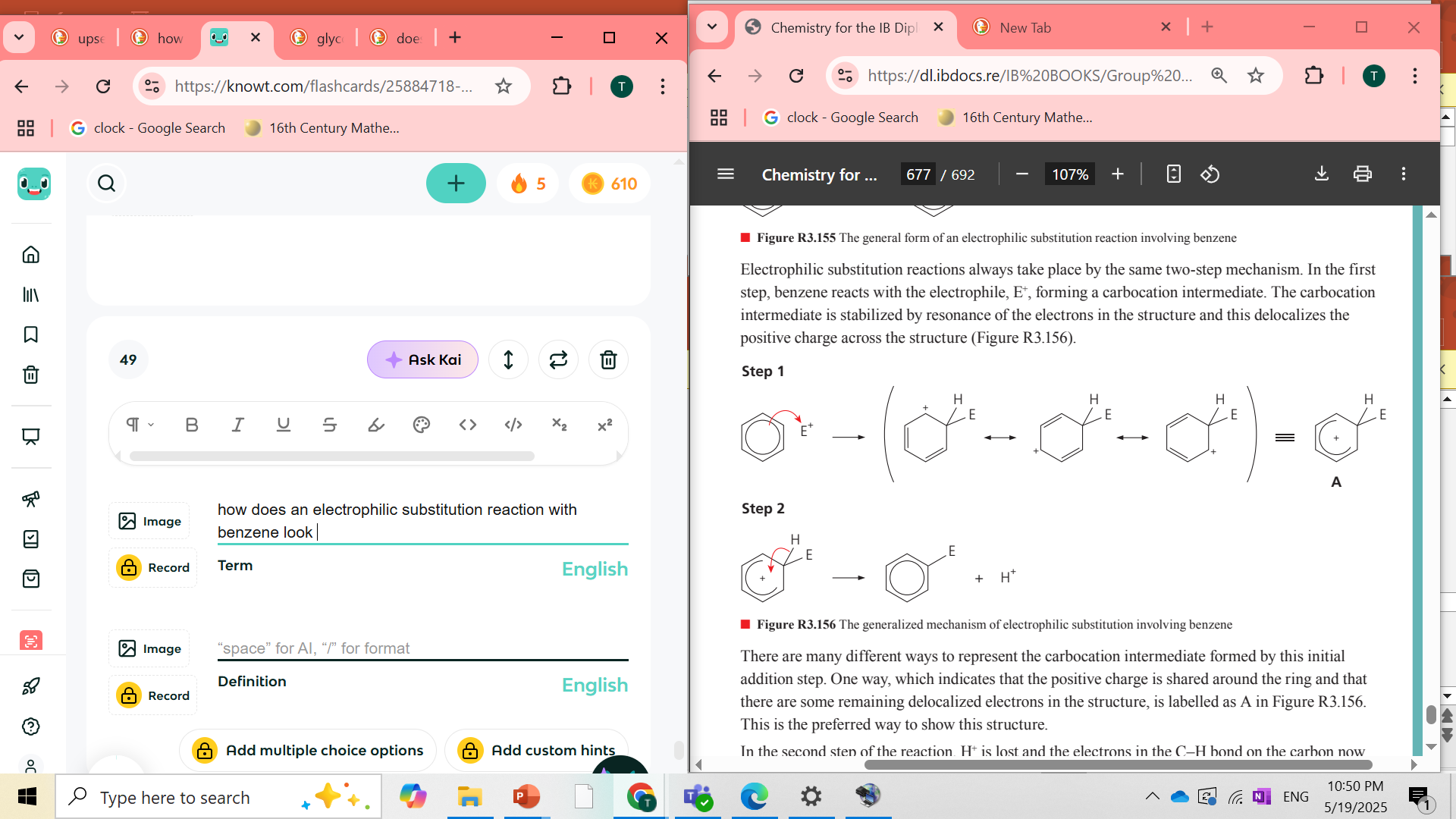
what happens to the delocalized e- when you bind an electrophile in order to do ESR
the resonance is disrupted and there is a positive carge where e- don’t pass through
which step in ESR with resonance is the slower one
the first one because benzene, a stable molecule is becoming unstable intermediate. it has high activation energy barrier (high energy energy transition state)