Final Exam Studying
1/7
Earn XP
Description and Tags
Chapters 6-7
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
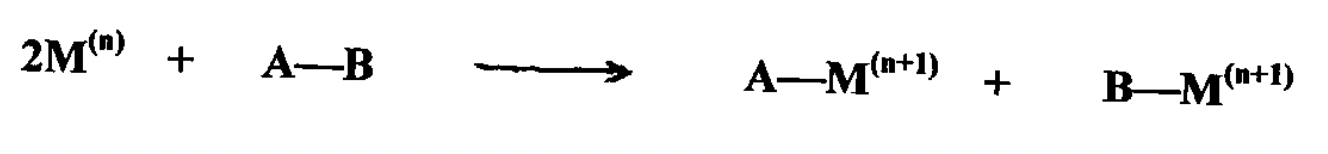
What type of oxidative addition can binuclear M complexes undergo?
1 electron oxidation
What is the order of selectivity for C-H oxidative addition of alkanes?
Primary > Secondary >> Tertiary
What type of CC bonds can undergo oxidative addition?
Strained CC bonds, such as cyclopropane
CC bonds when there is another L that can nucleophilically attack a C (ie: a Cp ligand)
Are alkenes with EWG substituents more or less reactive towards oxidative coupling?
More reactive as the C’s are more partial + positive
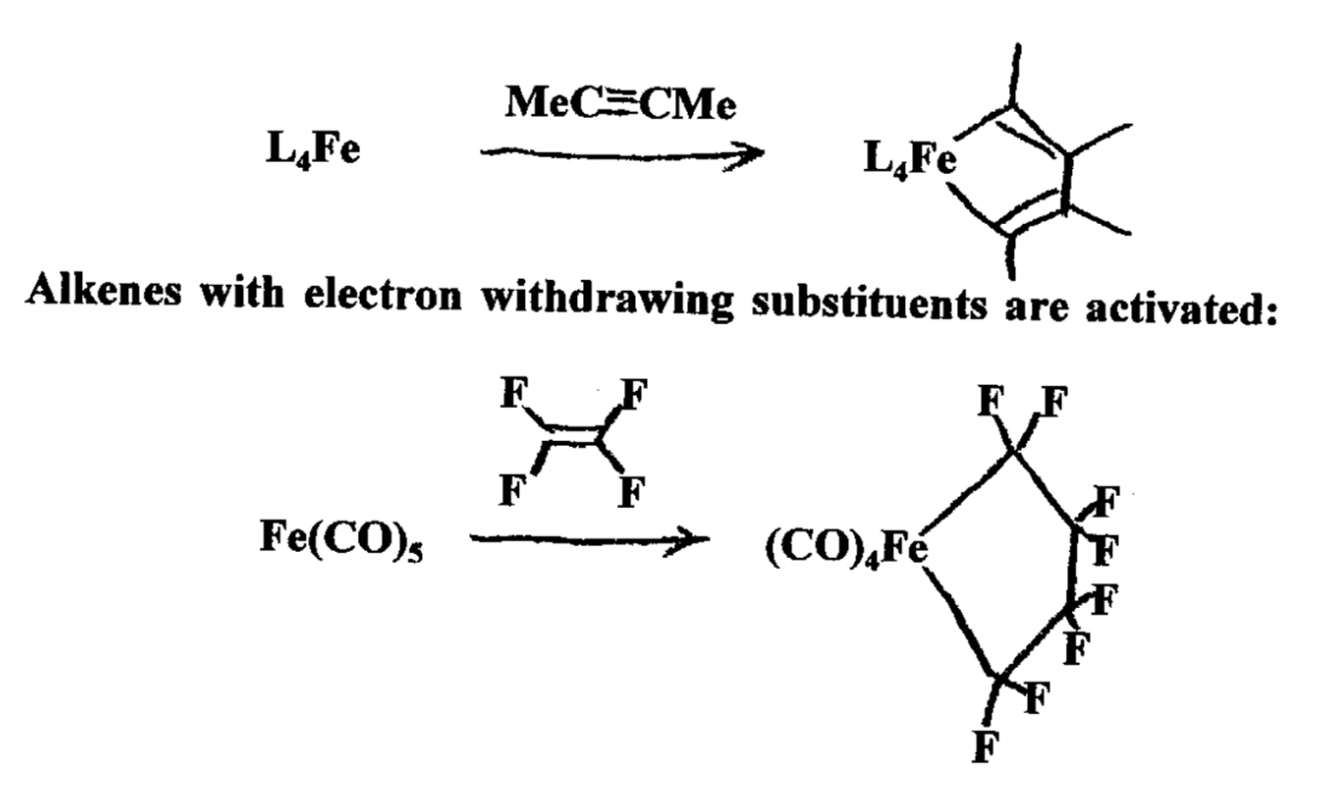
What is the relationship of pi-basicity of the M center and reactivity for oxidative coupling?
Strong pi-basicity —> higher reactivity for oxidative coupling
Does dissociation of sigma donor L’s increase or decrease reductive elimination reaction rate?
Increases reaction rate as it decreases the electron density of the M center
Are 4 coordinate d8 complexes reactive for reductive elimination?
No, must become 5 or 3 coordinate first
Is deuterated alkanes faster or slower for reductive elimination?
Deuterated alkanes are faster for reductive elimination