Bio. 1650, The Unity of Life Unit 2
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Hydrophobic
“Water-fearing”; non-polar molecules do not have intermolecular interactions with water
Hydrophilic
“Water-loving”; polar or ionic molecules easily from intermolecular forces with water
Phospholipid bilayer could form spontaneously
hydrophobic tails will move together to get away from water and the hydrophilic heads will move towards the water and of hydrogen bonds with the water thus creating a bilayer
Energy storage molecules forming large clusters inside a cell without a membrane-bound
Hydrophobic molecules will cluster together to avoid being around water, creating large groupings
Cohesion
The attraction of identical molecules for one another
Adhesion
The attraction of different moleclues
Surface Tension
Cohesion between water molecules is the force that generates surface tension
Xylem
The tissue in which water and minerals are transported from the roots to the leaves
Stomata
The structure of a plant that transpiration occurs
Transpiration
The evaporation of water from plant leaves and steams, driven by heat from the sun, and providing the motive force to raise water from the roots through the xylem. Does not require ATP
Transpiration-Cohesion-Tension Theory
Transpiration causes evaporation from mesophyll cell walls, generating tension on the xylem. Cohesion among water molecules in the xylem transmits the tension from the leaf to the root.
What do plants do to prevent the loss of too much water from their leaves
It closes the stomata and stops photosynthesis
Dissolve
The process of a molecule or atom/ion coming incorporated into liquid, such that the chemical is now “mixed” with the liquid and will not go back to its previous “undissolved” state
Solvent
A chemical that can dissolve a solute
Solute
A molecule or ion that dissolves in a solvent to form a solution.
Solution
The mixture of a solute dissolves into a solvent
Soluble (Solubility)
The ability of a molecule or ion to be solute and dissolve in a given solvent
Hydrogen Ion
Also called a proton, because a hydrogen atom consist of one proton and one electron, when the electron is removed, all that is left is a proton
HCI → H+ and Cl-
Adding HCI will increase the amount of H+ ions in solution.
HaOH → NA+ and OH-
OH- can interact with H+ to create H2O
Acid
A chemical that increases the amount of H+ ions in a solution and decreases the pH level
Base
A chemical that decrease the amount of H+ cons in a solution and raises the pH value
Homeostasis
The ability to use buffers to regulate or maintain a ~constant pH despite changes in surrounding pH
Chemical bonds
They can be considered a form of energy
First Law of Thermodynamics
Energy cannot be created or destroyed, only transformed from one form to another; the total amount of energy in a closed system is conserve (stays constant)
Second Law of Thermodynamics
When there is a transfer of energy, entropy increases in a closed system, meaning that energy becomes more disordered.
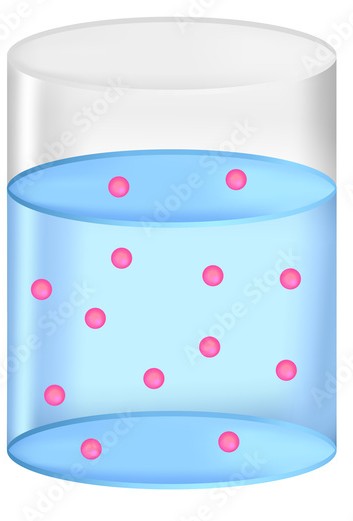
Concentrated
low entropy
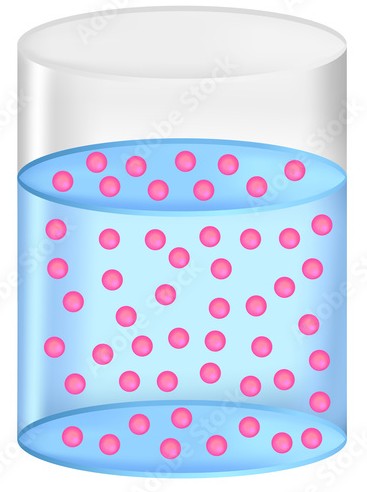
Equilibrium
highest entropy
Active energy
requires an input of ATP energy to occur, transport reverses or decreases entropy and creates more order
Passive energy
doesn’t require ATP energy to occur, transport increases entropy of the molecules
Activation energy
the minimum energy required to start a chemical reaction.
Reaction rate
the speed at which a chemical reaction occurs, often influenced by factors such as temperature, concentration, and catalysts.
Ligand
a molecule or ion that binds to another molecule; highly specific causes a change in the shape of a protein thus altering a proteins function