Chapter 2: The Chemistry of Life >
0.0(0)
Card Sorting
1/21
Last updated 2:51 PM on 12/6/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
1
New cards
Atom
a basic unit of matter
2
New cards
Nucleus
the central core of an atom
3
New cards
Electron
a negatively charged particle surrounding the nucleus
4
New cards
Element
a pure substance; made of only one type of atom
5
New cards
Isotope
a form of an element consisting of the same protons, but varying neutrons; different atomic mass
6
New cards
Compound
one type of molecule made up of more than one element
7
New cards
Ionic bond
a chemical bond formed from the exchange of electrons
8
New cards
Ion
an atom with a positive or negative charge
9
New cards
Covalent bond
a kind of bond between atoms formed from shared electrons
10
New cards
Molecule
two or more atoms joined together; the smallest particle of a substance
11
New cards
Van der Waals forces
A weak attraction between molecules that is dependent on distance, geckos use this to temporarily stick on walls.
12
New cards
Mass number
Protons and neutrons added
13
New cards
Atomic mass
the weighted average of the isotopes of an element
14
New cards
Atomic number
the number of protons in an atom
15
New cards
Weak Interactions
weak forces which create a slight attraction between molecules when they are close together
16
New cards
Radioactive Isotopes
The nuclei of the isotope are unstable, so they will break down at a constant rate over time.
17
New cards
Max number of electrons in a valence shell
eight
18
New cards
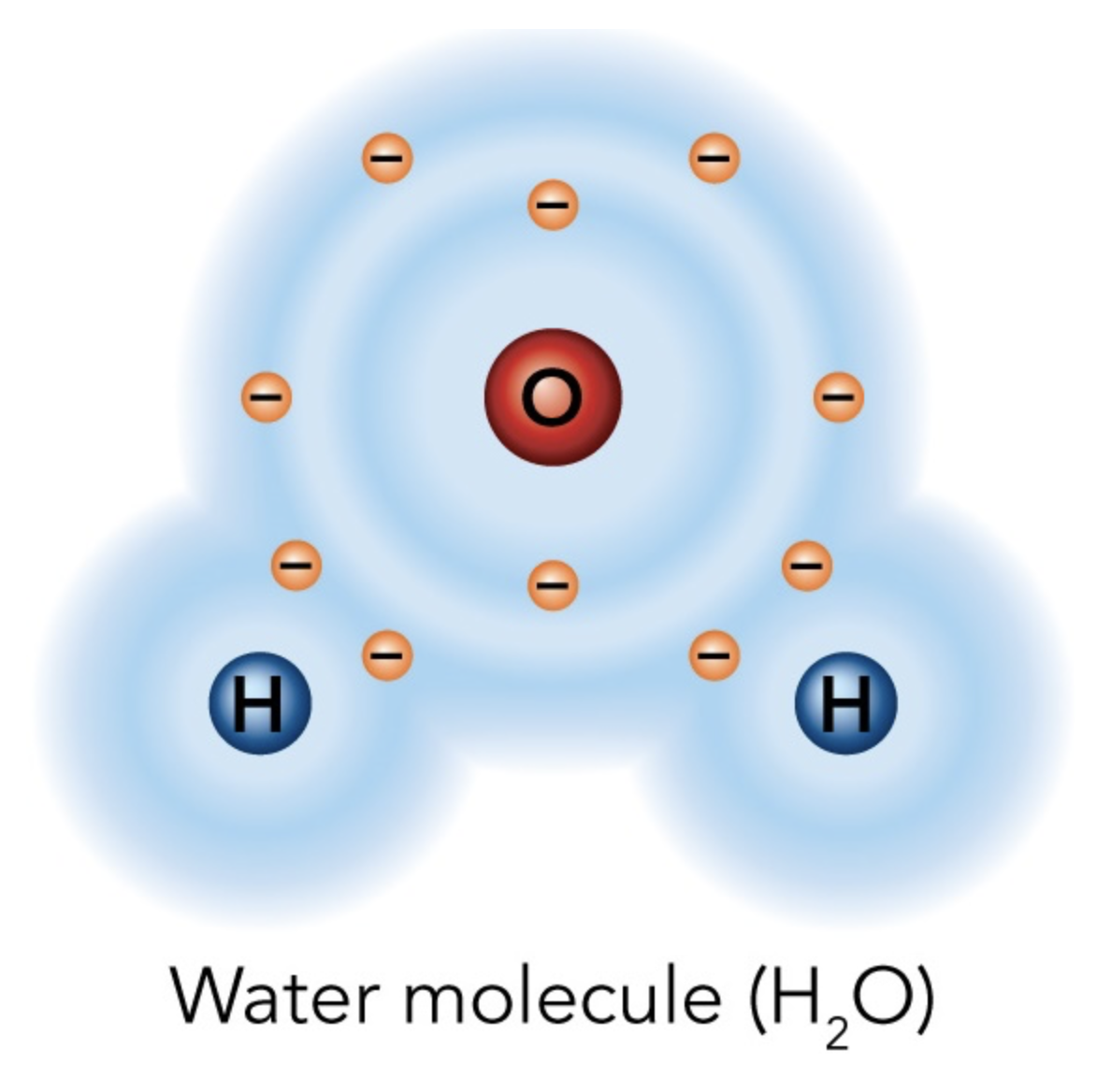
What kind of bond does this diagram contain?
Covalent bond
19
New cards
Trace element
A chemical element present in a minuscule quantity
20
New cards
6 most common elements in living things?
carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus
21
New cards
99% of the human mass's elements are...
oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus
22
New cards
AMU
Atomic Mass Unit