orgo I rxns
1/21
Earn XP
Description and Tags
ch 6-9
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
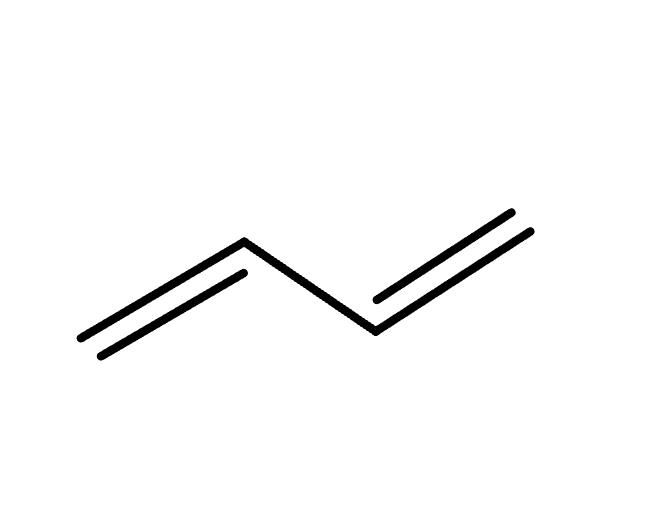
reagents: H-Nu (addition of H2O, HX, or ROH)
mechanistic notes: conjugate addition often thermodynamic product, direct addition is kinetic product
notes: 1,4-addition (conjugate), competes w/ 1,2-addition (direct)
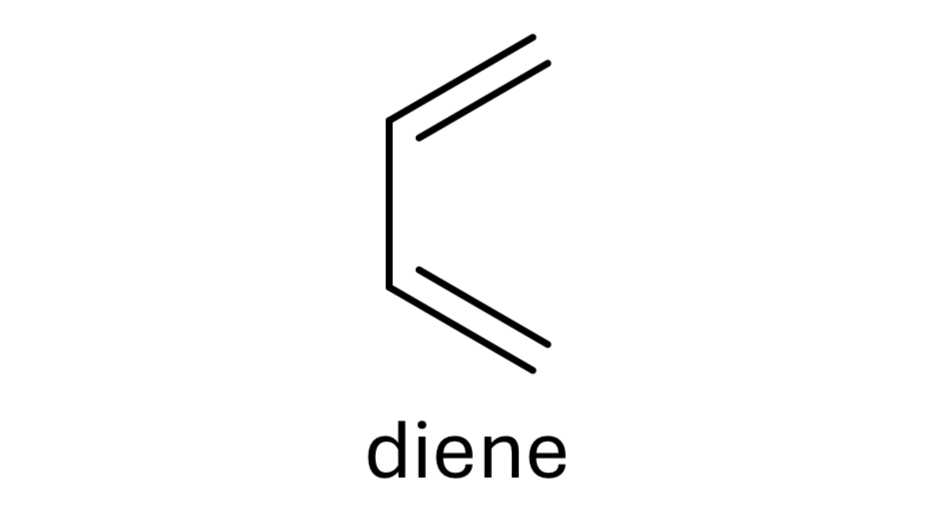
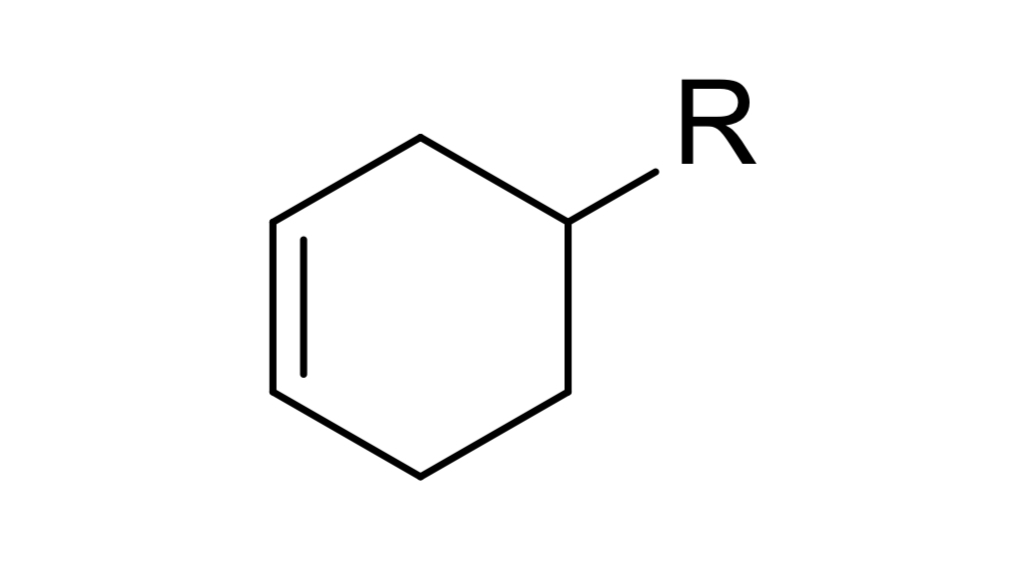
reagent: dienophile
mechanistic notes: regiochemistry determined by EWG & EDG, always forms 6 membered ring
notes: diels-alder rxn, ____
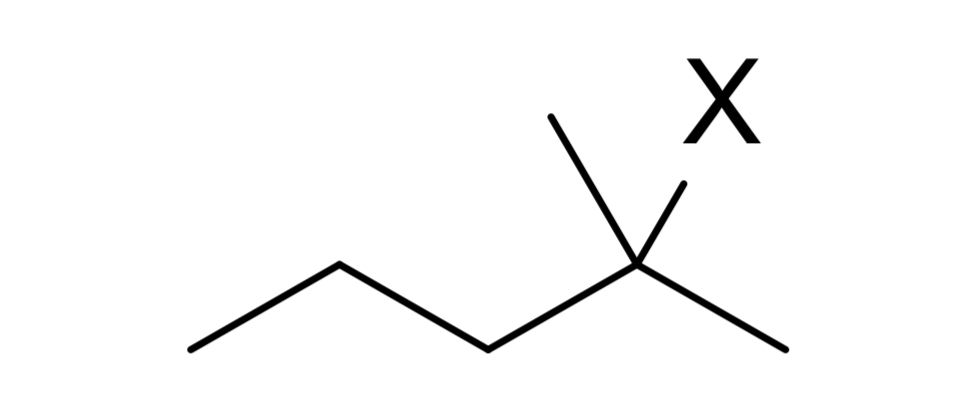
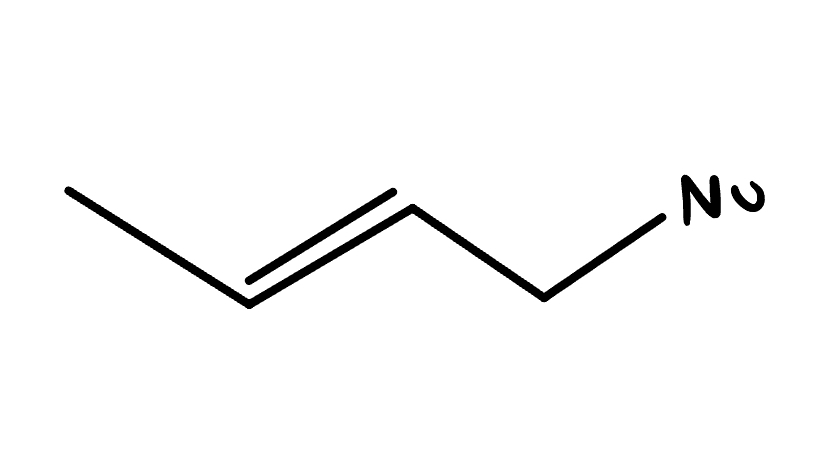
reagent: neutral nucleophile (ends w Nu)
mechanistic notes: racemic product, solvents like H2O, ROH, RNH2 (neutral amines) as nucleophile
notes: SN1, occurs w/ E1, favored at lower temps
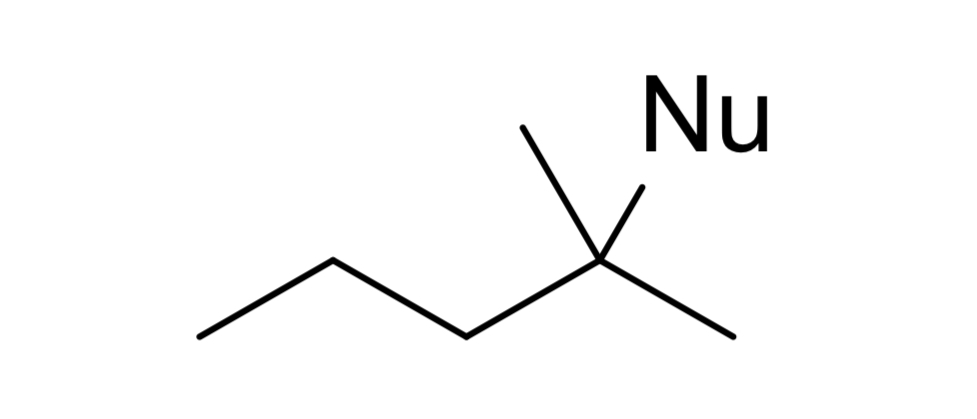
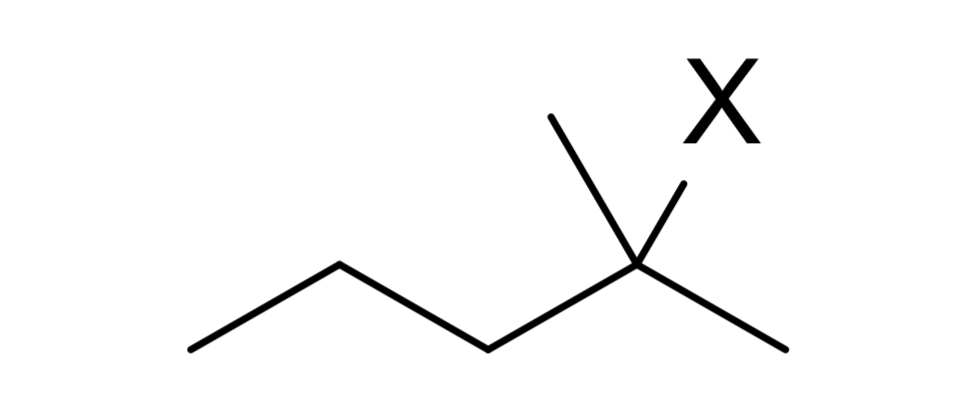
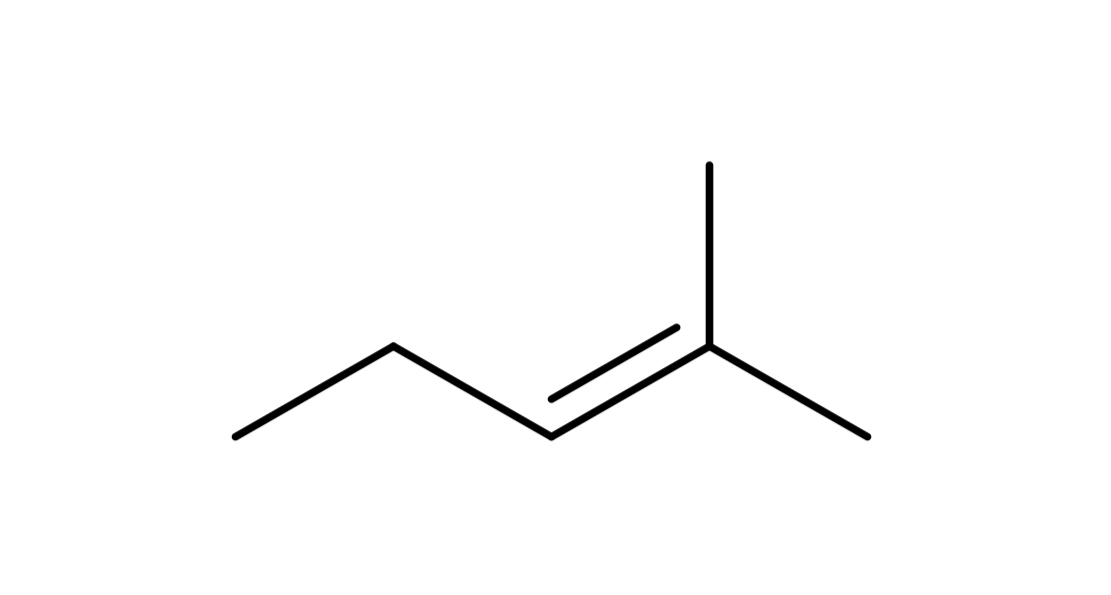
reagent: neutral nucleophile (ends w double bond)
mechanistic notes: always zaitsev product, requires beta H, solvents like H2O, ROH, RNH2 (neutral amines) as nucleophile
notes: E1, occurs w/ SN1, favored at higher temps
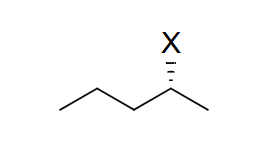
reagent: strong nucleophile (charged)
mechanistic notes: inversion of stereochemistry, always favored for primary alkyl halides, favored for secondary if weak base (NaCN, NaSH)
notes: SN2, may occur w/ E2
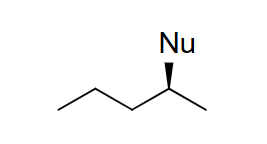
reagent: strong base (charged)
mechanistic notes: hofmann if large strong base (tBuOH), zaitsev if small strong base, always favored for tertiary alkyl halides, favored for secondary if strong base
notes: E2, can occur w/ SN2, favored at higher temps
reagents: HCl, HBr, HI (1 eq)
regiochemistry? markovnikov (X to more substituted carbon)
stereochemistry? none
notes: excess reagent forms geminal dihalide

reagents: Cl2, Br2 (organic solvent) (1 eq)
regiochemistry? N/A
stereochemistry? anti-addition, trans halogenation
notes: excess reagent forms tetrahalide

reagents: H2O, H2SO4
regiochemistry? markovnikov (OH to more substituted carbon)
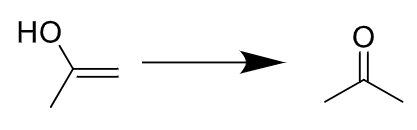
stereochemistry? N/A, pdt immediately tautomerizes
notes: makes ketone final pdt, terminal alkenes need HgSO4 catalyst
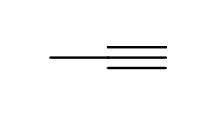
reagents: 1. 9-BBN, THF
-OH, H2O2, H2O
regiochemistry? anti-markovnikov (OH to less substituted carbon)
stereochemistry? N/A, pdt immediately tautomerizes
notes: makes ketone or aldehyde final pdt
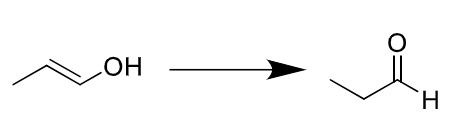
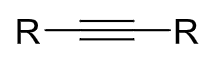
reagents: H2, Pd/C
regiochemistry? N/A
stereochemistry? none
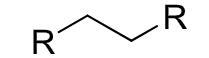
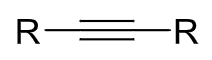
reagents: H2, Lindlar’s catalyst
regiochemistry? N/A
stereochemistry? cis hydrogenation
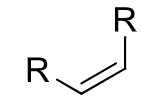

reagents: Na or Li, NH3
regiochemistry? N/A
stereochemistry? trans hydrogenation
reagents: HCl, HBr, HI
regiochemistry? markovnikov (Cl to more substituted carbon)
stereochemistry? none
carbocation rearrangements? yes

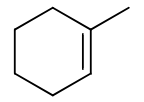
reagents: H2O, H2SO4
regiochemistry? markovnikov (OH to more substituted carbon)
stereochemistry? none
carbocation rearrangements? yes
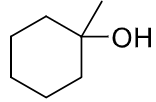
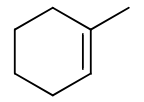
reagents: ROH, H2SO4
regiochemistry? markovnikov (OR to more substituted carbon)
stereochemistry? none
carbocation rearrangements? yes
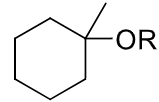
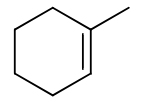
reagents: 1. BH3, THF
-OH, H2O2, H2O
regiochemistry? anti-markovnikov (OH to less substituted carbon)
stereochemistry? syn-addition
carbocation rearrangements? no
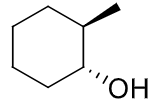
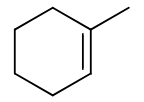
reagents: Cl2, Br2 (organic solvent)
regiochemistry? none
stereochemistry? anti-addition
carbocation rearrangements? no
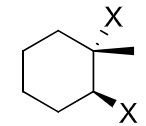
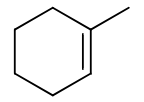
reagents: Cl2, Br2 (water or alcohol as solvent)
regiochemistry?
stereochemistry?
carbocation rearrangements? no
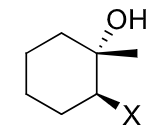
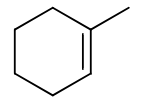
reagents:
regiochemistry?
stereochemistry?
carbocation rearrangements? no
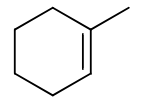
reagents:
regiochemistry?
stereochemistry?
carbocation rearrangements? no
reagents:
regiochemistry?
stereochemistry?
carbocation rearrangements? no