VCE Biology - Unit 3 AOS 1
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
Biomacromolecule
a large organic molecule, found in organisms
Protein
a biomacromolecule made of amino acids folded in a 3D shape
Polypeptide
a long chain of amino acids
Proteome
all the proteins that are expressed by a cell or organism at a given time
Some of the diverse functions of proteins are...
1. Enzymes
2. Transport
3. Structural
4. Hormones
5. Receptors
6. Defence
7. Motor/contractile
8. Storage
An amino acid molecule is composed of...
a central carbon bonded to a hydrogen atom, a carboxyl group, an amino group and an R-group
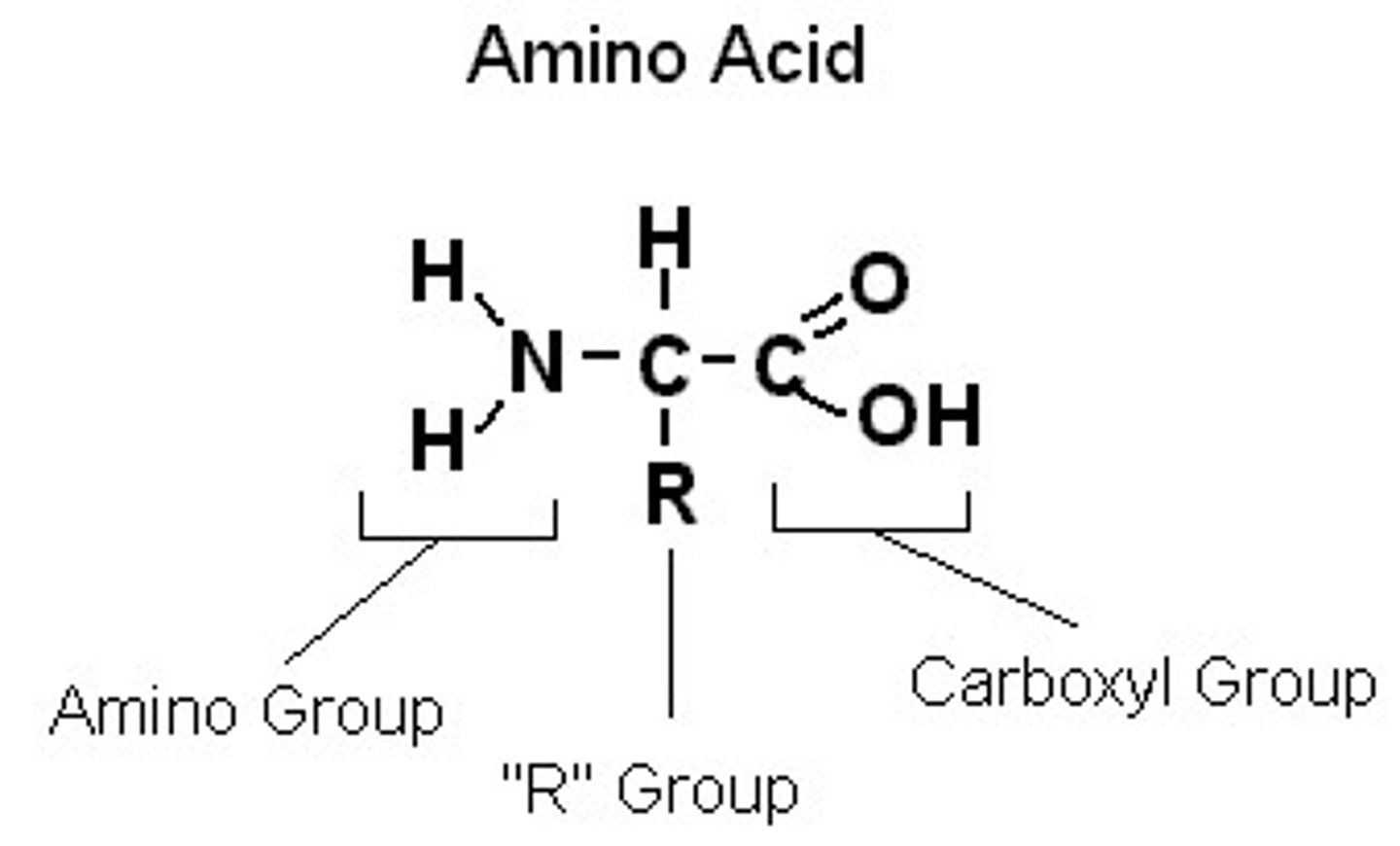
R-group
the variable portion of an amino acid molecule that determines that identity of the amino acid
Fill in the blank: An amino acid with a hydrophobic R-group is _______ likely to form bonds with another amino acid with a hydrophobic R-group
more
Fill in the blank: An amino acid with a hydrophilic R-group is _______ likely to form bonds with another amino acid with a hydrophilic R-group.
more
What are the monomers of proteins?
amino acids
Condensation reaction
a reaction where two monomers join to form a larger molecule, producing water as a by-product
The chemical bond linking two amino acids
peptide bond
Primary structure
the sequence of amino acids in a polypeptide chain
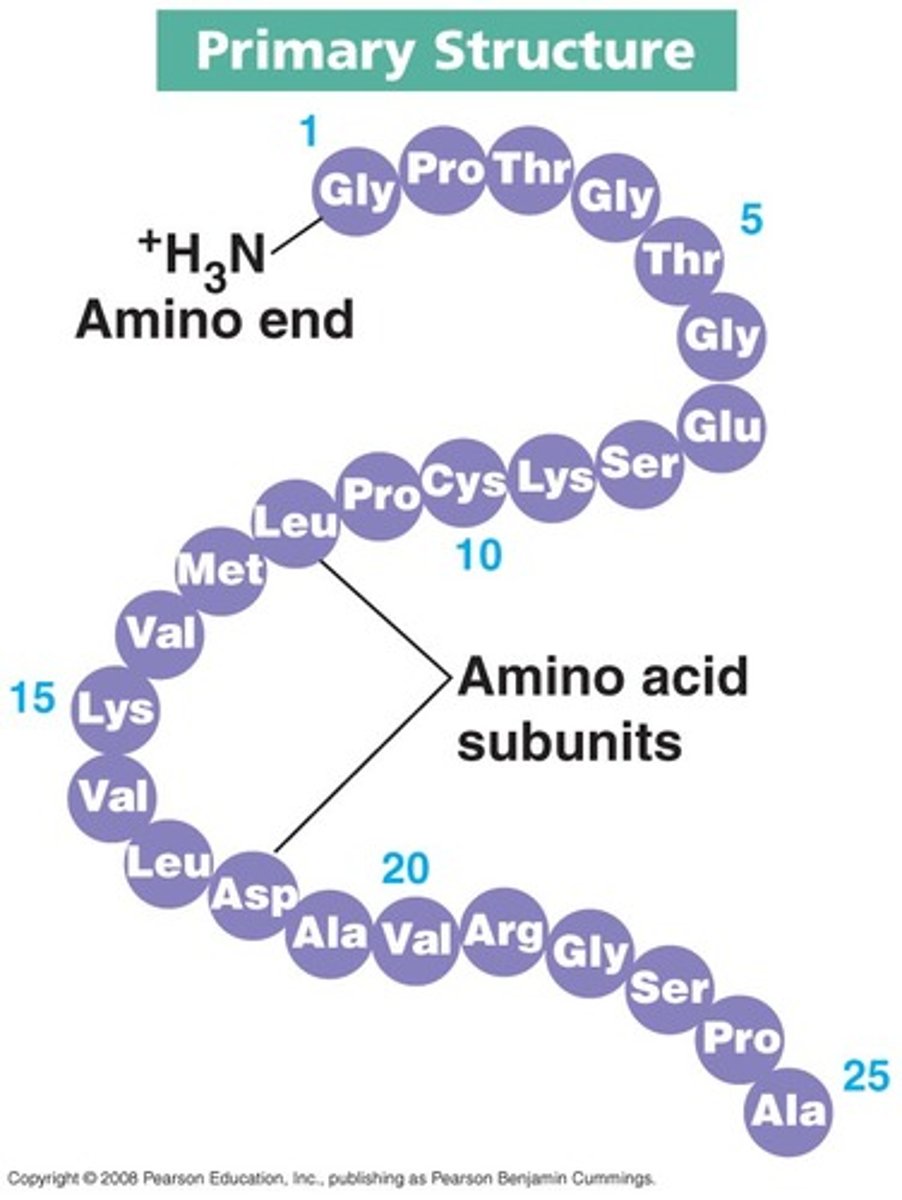
Secondary structure
the second level of protein structure where an amino acid chain forms either alpha-helices, beta-pleated sheets or random coils
Tertiary structure
The functional 3D shape of a polypeptide chain
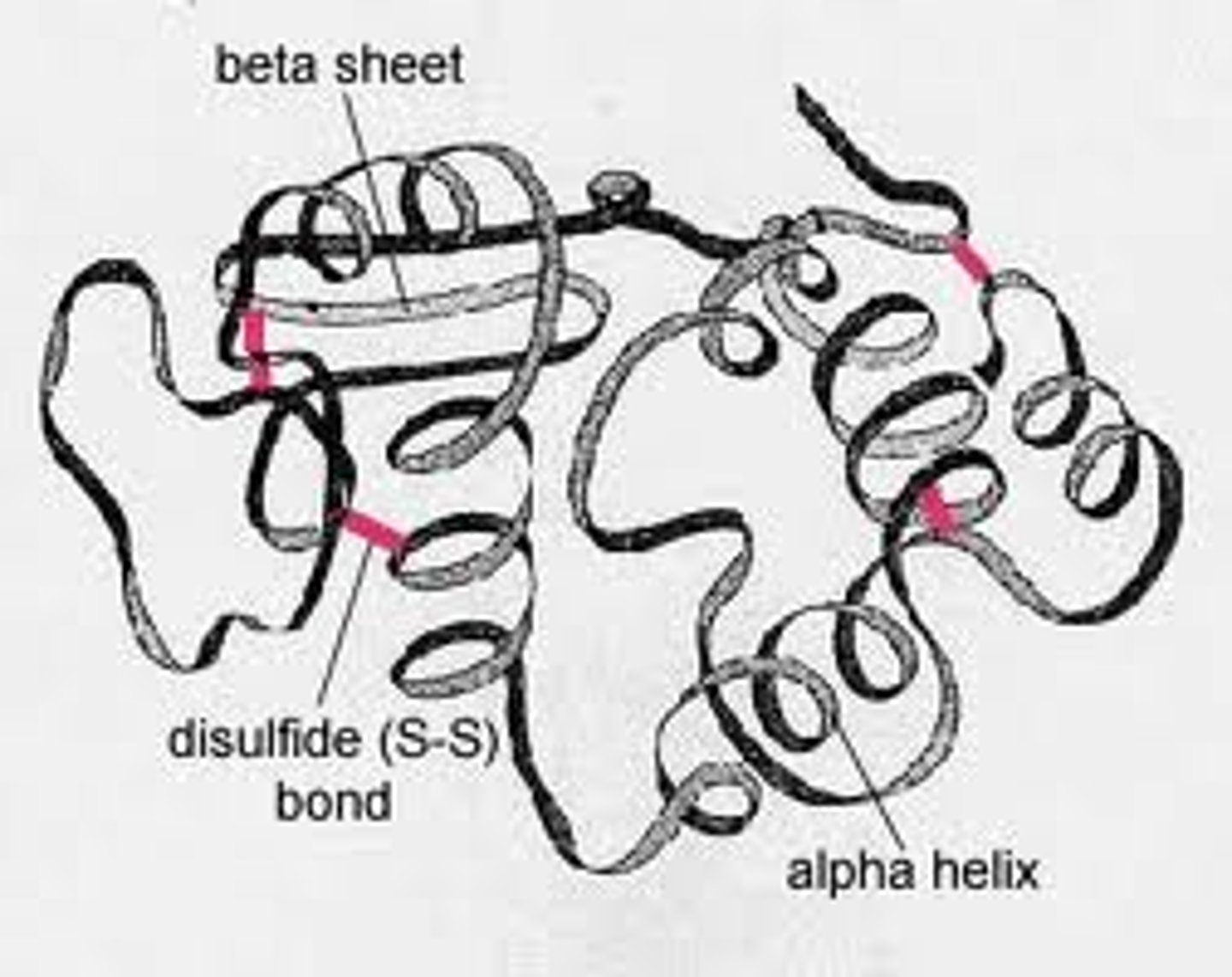
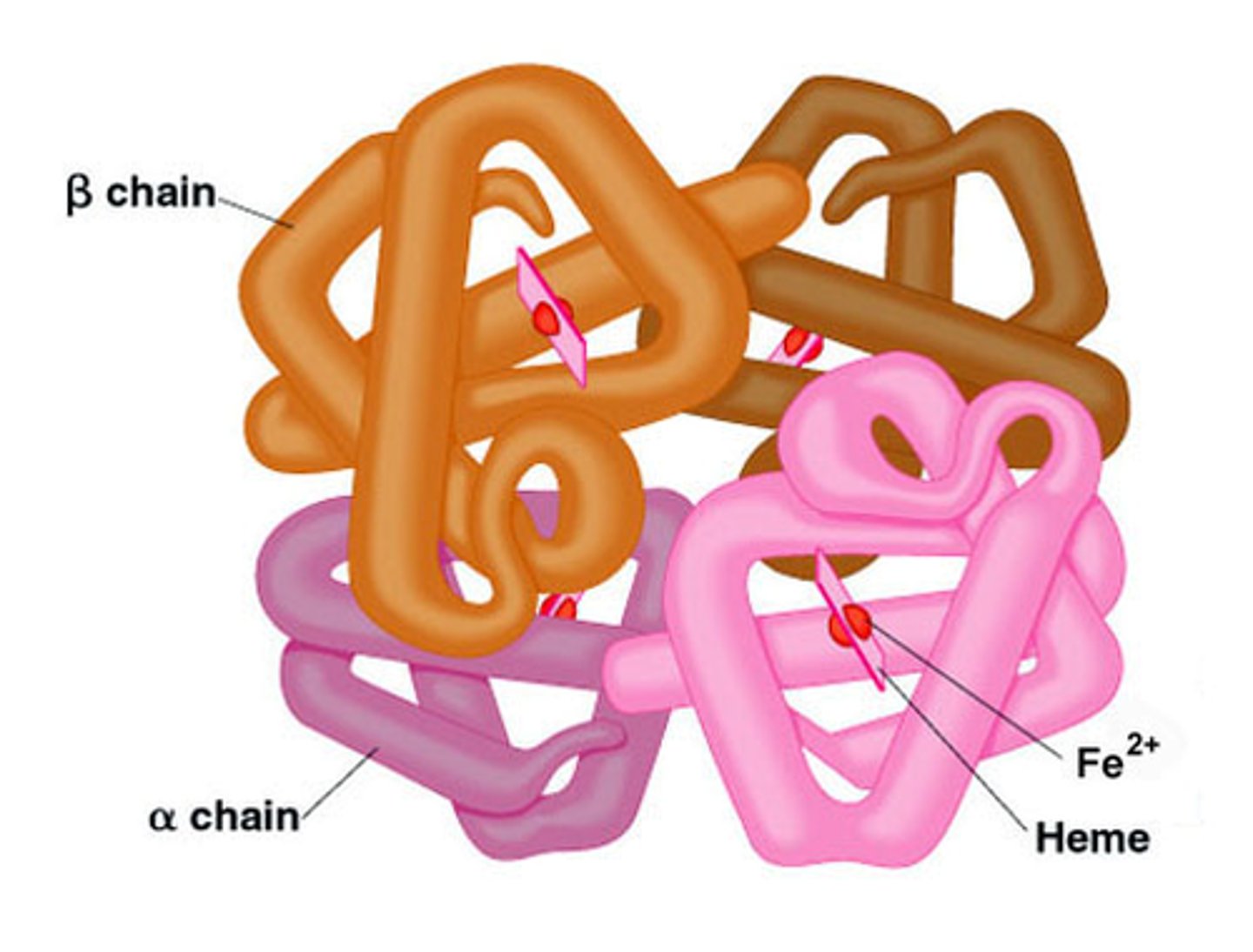
Quaternary structure
The level of protein structure where multiple polypeptide chains bond together to form a fully functional protein
Nucleic acid
Large polymers composed of nucleotide monomers - includes DNA and RNA
Nucleotide
The monomer subunit of nucleic acids
What is every nucleotide composed of?
A phosphate group, a five-carbon (pentose) sugar and a nitrogenous base
DNA is...
a double-stranded nucleic acid made up of nucleotides and carries the instructions for the production of proteins
RNA is...
a single-stranded nucleic acid chain made up of nucleotides (mRNA, rRNA, tRNA)
Phosphodiester bond (in terms of nucleotides)
the strong covalent bond that links the pentose sugar and the phosphate group in the sugar-phosphate backbone
The complementary base pairings in DNA
Adenine (A) to Thymine (T) and Cytosine (C) to Guanine (G)
Genome
the complete set of DNA contained within an organism
Gene
a section of DNA that carries the code to make a protein
Antiparallel
a characteristic of DNA describing how each strand runs in an opposite direction to the other. One strand runs in a 3' to 5' direction and the other runs in a 5' to 3' direction
How many hydrogen bonds exist between A and T?
two
How many hydrogen bonds exist between G and C
three
In what structure is DNA stored?
in a double helix
Fill in the blank: in a double helix, there are ______ base pairs per complete twist
10.5
messenger RNA (mRNA)
RNA molecules that are produced during transcription and carry genetic info from the nucleus to the ribosomes
transfer RNA (tRNA)
RNA that recognises specific codons on the mRNA strand and adds the corresponding amino acid to the polypeptide chain during protein synthesis
ribosomal RNA (rRNA)
RNA that is a key structural component of ribosomes
What is the role of ribosomes in protein synthesis?
to assemble proteins
What are the four differences in the structures of DNA and RNA
- DNA is double stranded, whereas RNA is single stranded
- DNA contains a deoxyribose sugar, whereas RNA contains a ribose sugar
- DNA contains thymine, whereas RNA contains uracil
- DNA is inherited and is used for long-term storage of genetic information, whereas RNA is temporary and short-lived
Genetic code
the set of rules which information is encoded in genetic material
Triplet
the sequence of three nucleotides in DNA that codes for one amino acid
Codon
the sequence of three nucleotides in mRNA that codes for one amino acid
Universal (genetic code property)
all organisms have the same codons to code for specific amino acids
Unambiguous (genetic code property)
each codon only codes for one amino acid
Degenerate (genetic code property)
each amino acid can be coded for by multiple codons
Non-overlapping (genetic code property)
each triplet or codon is read independently
Promoter region
an upstream binding site for RNA polymerase on the 5' end of a gene
Introns
the non-coding regions of DNA (only in eukaryotic cells)
Exons
the regions of DNA that code for proteins
Termination sequence
a sequence of DNA that signals the end of transcription
Operator region
the binding site for repressor proteins (typically only found in prokaryotic cells)
Leader region
the segment of DNA or mRNA that immediately precedes the coding region and plays a role in the regulation of gene expression in prokaryotes
Explain the process of transcription
- DNA is unwound by helicase and RNA polymerase binds to the promoter region of the gene's template strand
- RNA polymerase runs along the template (coding) strand of DNA, reading the nucleotide sequence and building a pre-mRNA strand using free-floating complementary RNA nucleotides
- When RNA polymerase reaches the termination sequence of a gene, transcription is terminated
What modifications are made to a pre-mRNA molecule during RNA processing?
the addition of a 5' methyl-guanine cap and a 3' poly-A tail, as well as the splicing (removal) of introns and the annealing (joining) of exons
What enzyme removes introns and anneals exons from pre-mRNA during RNA processing?
Spliceosome
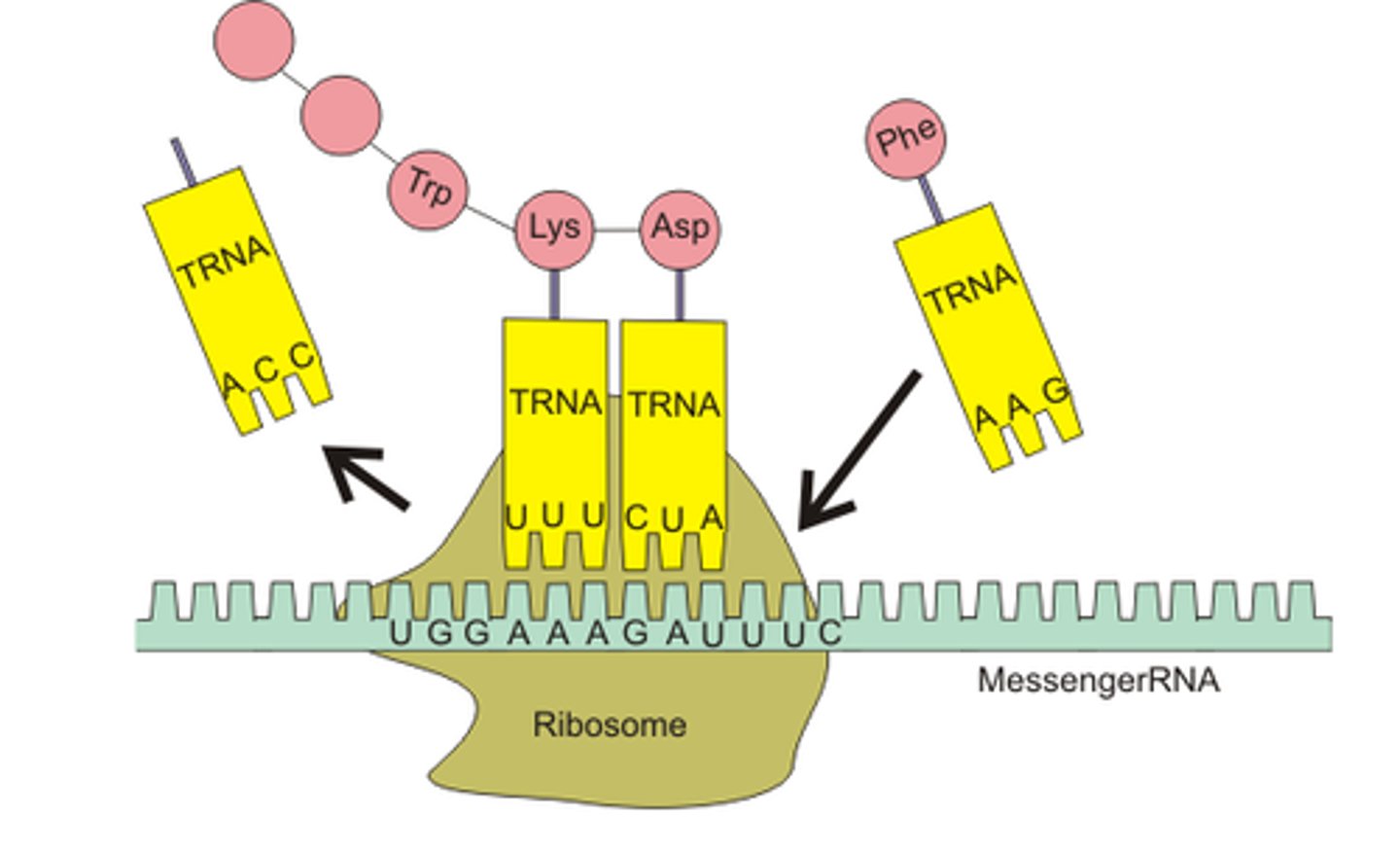
Explain the process of translation
- The 5' end of the mRNA molecule binds to a ribosome in the cytoplasm
- The ribosome reads the mRNA molecule until the start codon is reached, signalling the start of translation
- tRNA anticodons complementary to mRNA codons deliver corresponding amino acids to the ribosome, and each amino acid is added to the polypeptide chain via a peptide bond with an adjacent amino acid via a condensation reaction
-Once a stop codon is reached, translation is terminated and the polypeptide chain produced is released from the ribosome
If a protein is to be transported out of the cell, what steps occur?
- Once released by the ribosome, a polypeptide chain moves to the rough E.R, where the polypeptide chain folds into a functional structure (tertiary or quaternary)
- The protein is then transported to the Golgi apparatus via a transport vesicle that buds off the rough E.R
- The Golgi apparatus modifies the protein, and it is packaged into a secretory vesicle which buds off the Golgi apparatus
- The secretory vesicle containing the protein then travels to and fuses with the plasma membrane, releasing the protein in the extracellular environment via exocytosis
Structural gene
codes for proteins that play a role in the structure and function of a cell
Regulatory gene
produces proteins that control the expression of other genes
Activator protein
a protein encoded for by a regulatory gene that increases gene expression if it binds to the operator region of an operon
trp operon
a series of genes within certain species of bacteria that encode for the production of the amino acid tryptophan
In terms of trp operon repression, what happens if tryptophan levels in a cell are high?
- the regulatory gene is transcribed
- tryptophan binds to a repressor protein, which induces a conformational change in the repressor protein, changing it into its active form
- the repressor protein then binds to the operator region of the trp operon, which blocks the path of RNA polymerase thus preventing the production of tryptophan
In terms of trp operon repression, what happens if tryptophan levels in a cell are low?
- the regulatory gene is transcribed
- no tryptophan is available to bind to a repressor protein, making it inactive and preventing it from binding to the operator region
- RNA polymerase is then able transcribe the trp operon structural genes and tryptophan is produced
What is an enzyme?
a biological catalyst
What is the main purpose of enzymes in a reaction?
to lower the activation energy required for the reaction to occur
What structure do most enzymes have?
a tertiary structure
Substrate
the reactant of an enzyme-facilitated reaction
Outline the general steps involved in an enzyme reaction
- the substrate binds to the active site of an enzyme that is complementary to its structure, and the enzyme's active site undergoes a conformational change to accommodate the substrate.
- after the reaction occurs, the products leave the active site and the enzyme remains unchanged, free to catalyse further reactions
Reusable feature of enzymes
when an enzyme catalyses a reaction, it is not consumed and can catalyse future reactions
Specific feature of enzymes
most enzymes only bind to one specific substrate
Reversible feature of enzymes
most enzyme-catalysed reactions can be reversed
Speed up, not create feature of enzymes
enzymes catalyse reactions, and don't create reactions
Have an active site feature of enzymes
each enzyme has an active site, the area to which is substrate binds and which is complementary to the substrate's structure
Are proteins feature of enzymes
most enyzmes are proteins
Are a subset of catalysts feature of enzymes
all enzymes are catalysts, but not all catalysts are enzymes
Act on entire biochemical pathways feature of enzymes
enzymes frequently influence biochemical pathways, catalysing each step
Naming of enzymes
Most enzymes end with the suffix '-ase' (e.g. catalase, ligase)
Above the arrow (feature of enzymes)
In a chemical equation, enzymes are typical displayed above the reaction arrow
Activation energy
the energy required to initiate a reaction
Catabolic reaction
an enzyme reaction that involves the breakdown of a molecule into multiple smaller molecules and releases energy (exergonic) (e.g. the breakdown of hydrogen peroxide into water and oxygen)
Anabolic reaction
an enzyme reaction that involves the formation of larger molecule from smaller molecules and absorbs energy (endergonic) (e.g. the joining of amino acids during protein synthesis to form a polypeptide)
Biochemical/metabolic pathway
a series of enzyme-catalysed biochemical reactions in which the product of one reaction becomes the substrate of the next reaction
What is an enzyme's optimum?
the point at which the maximum function of an enzyme occurs for a given condition
What happens to an enzyme when temperature is too high?
the enzyme denatures, causing an irreversible conformational change in the enzyme's active site. Due to this, the substrate can no longer bind to the active site, meaning that the enzyme permanently loses its function
What happens to an enzyme when temperature is too low?
Kinetic energy decreases significantly, which causes an enzyme to have minimal activity and freeze. However, this change is reversible (given that temperature returns to within the optimum) since denaturation does not occur and an enzyme retains functionality
What happens if the pH level is too basic or too acidic for an enzyme?
the enzyme will denature
Fill in the blank: if the substrate concentration increases and enzyme concentration remains constant, the reaction rate will ________
increase
Fill in the blank: if the enzyme concentration increases and substrate concentration stays constant, the reaction rate will ________
increase
Saturation point
the point at which all active sites are consistently occupied
Limiting factor
a reactant that prevents the reaction rate from increasing
How is the rate of an enzyme reaction calculated?
amount of substrate/time
Competitive inhibitor
a type of enzyme inhibitor that binds to the active site of an enzyme, blocking the active site and preventing the substrate from binding
Reversible inhibition
enzyme inhibition where the bonds between the inhibitor and the enzyme's active site are weak and can be overcome, slowing the rate of an enzyme reaction
Irreversible inhibition
enyzme inhibition where the bonds between the inhibitor and the enzyme's active site are strong and unbreakable, preventing the substrate from binding indefinitely
Non-competitive inhibitor
a type of enzyme inhibitor that binds to an allosteric site of an enzyme, causing a conformational change in the enzyme's active site and thus preventing the substrate from binding
Coenzyme
a non-protein organic cofactor that assists enzyme function by releasing energy/molecules, which are used in an enzyme reaction
To what part of an enzyme does a coenzyme bind?
the active site
What happens to coenzymes after they assist an enzymatic reaction?
they leave the enzyme and accept more energy, and can then go on to assist further enzyme reactions
Endonucleases
enzyme that break the phosphodiester bond between the pentose sugar and the phosphate of the sugar-phosphate backbone in a DNA strand (molecular scissors)
Restriction site
the sequence on a DNA molecule that endonucleases act upon
Restriction sites are...
typically palindromes, where the sequence on the 5' to 3' template strand is the same as the 3' to 5' non-template strand
Sticky end
overhanging, unbonded nucleotides that result from a staggered cut through a DNA strand by an endonuclease
Why are sticky ends useful for DNA manipulation purposes?
sticky ends are attracted to complementary nucleotides, so an inserted gene can be orientated correctly
Outside of DNA manipulation, why might sticky ends be an issue?
as the presence of unbonded nucleotides means that the DNA strand is not complete.