Labmath and Graphing
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
CFU
Colony forming unit
Make 1L of a 0.9% (w/v) saline solution
know that 1g = 1 ml; know that 1L = 1000 ml; know that saline is made from NaCl (salt); w/v = weight/volume percent = measures the amount of solute in grams but measures the amount of solution in milliliters; cross multiply to get answer: 1000 ml = 100%; x ml = 0.9%; x= (1000 ml x 0.9%)/100% = 9 g
CFU/ml
Colony forming unit/ml - written in scientific notation
Aliquot
a portion of a larger whole, especially a sample taken for chemical analysis or other treatment.
C1 x V1 = C2 x V2
Formula for calculating dilutions and concentrations
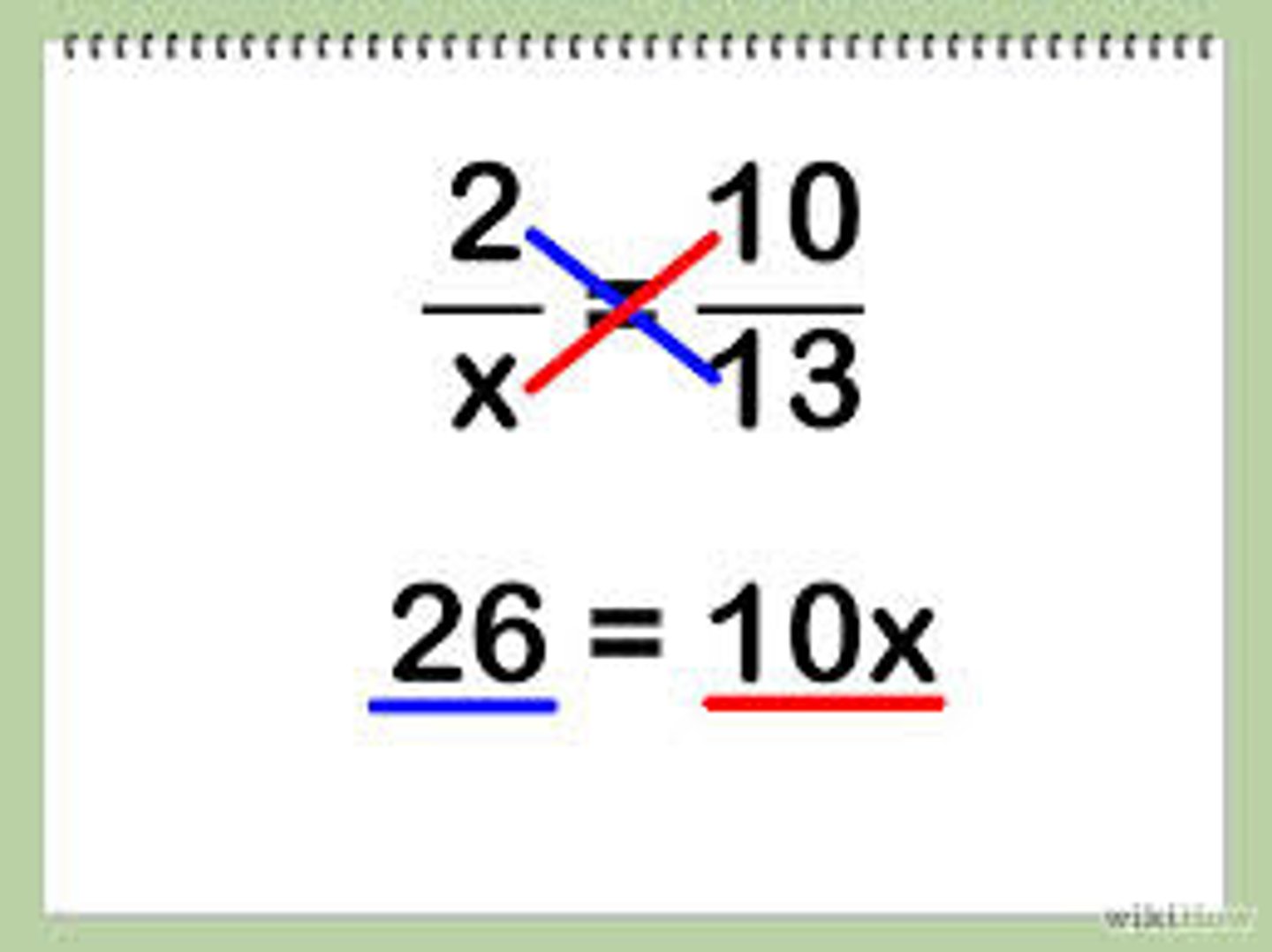
Cross multiplication
Solve for the unknown (x) by cross-multiplication
Diluent
a substance used to dilute something
Dilution
the action of making a liquid more dilute
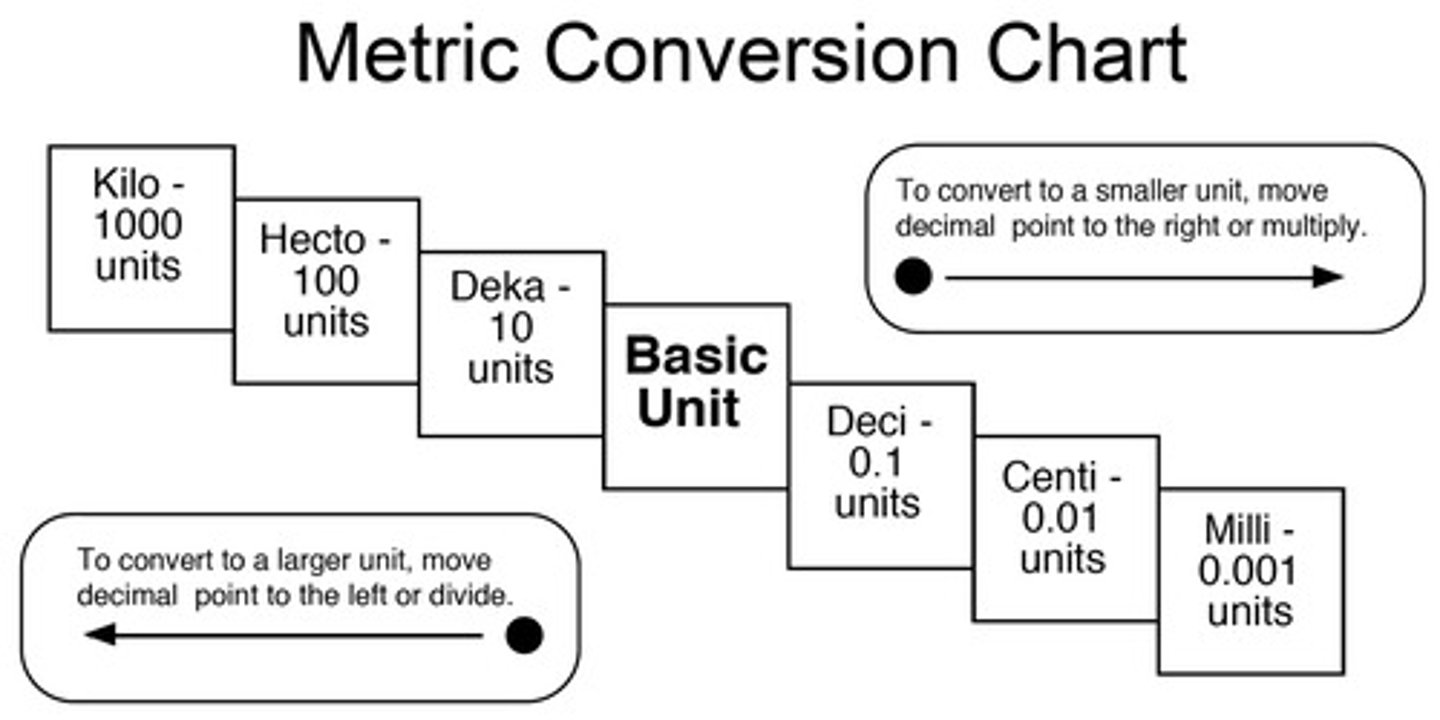
Metric system
the decimal measuring system based on the meter, liter, and gram as units of length, capacity, and weight or mass. The system was first proposed by the French astronomer and mathematician Gabriel Mouton (1618-94) in 1670 and was standardized in France under the Republican government in the 1790s
Molar
Molar concentration, also called molarity, amount concentration or substance concentration, is a measure of the concentration of a solute in a solution, or of any chemical species, in terms of amount of substance in a given volume. A commonly used unit for molar concentration used in chemistry is mol/L. A solution of concentration 1 mol/L is also denoted as 1 molar (1 M). 1 molar soln=1mole of solute
Ratio
A ratio says how much of one thing there is compared to another thing. Example: 1 part stock solution and 4 parts diluent (1:4)
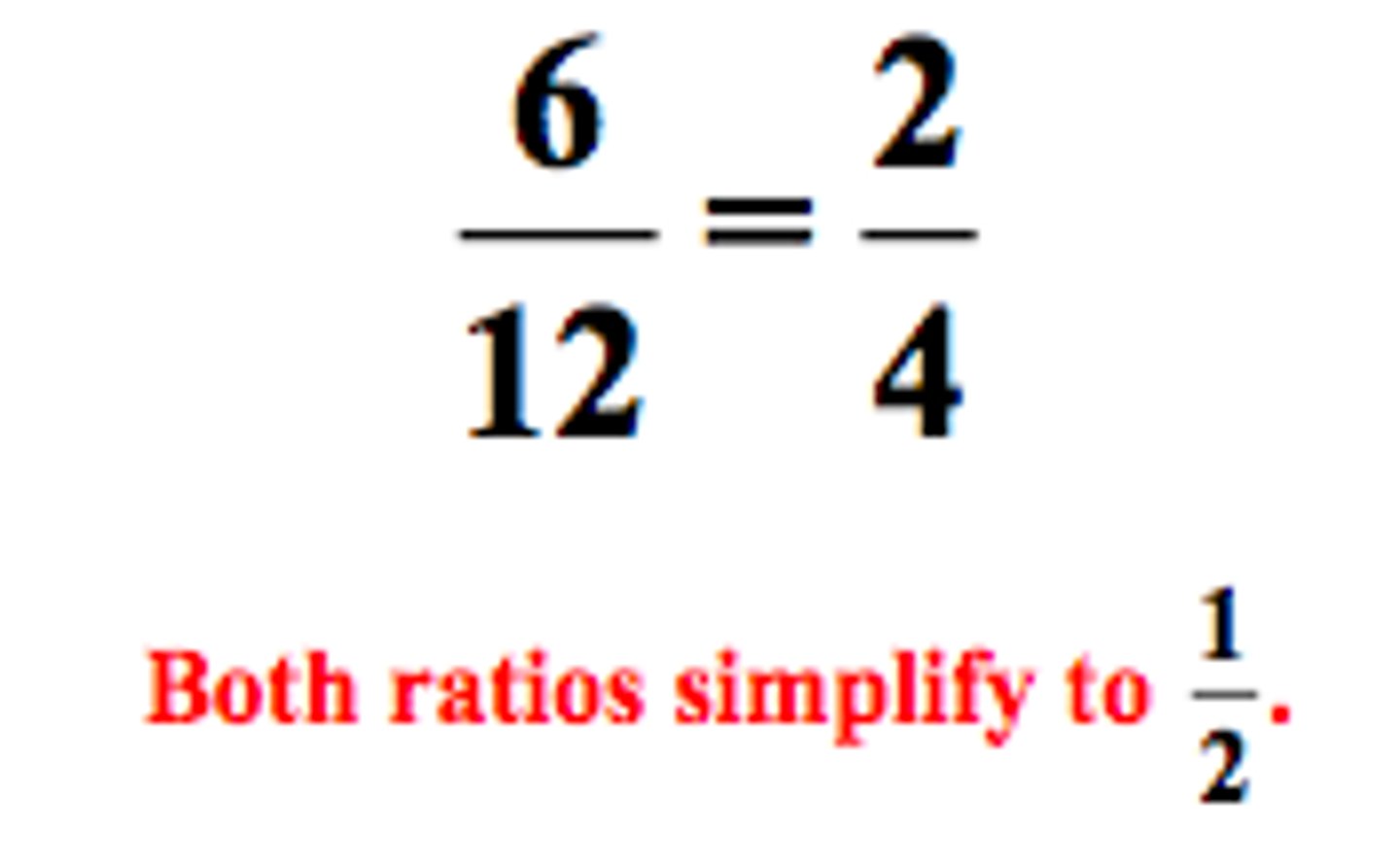
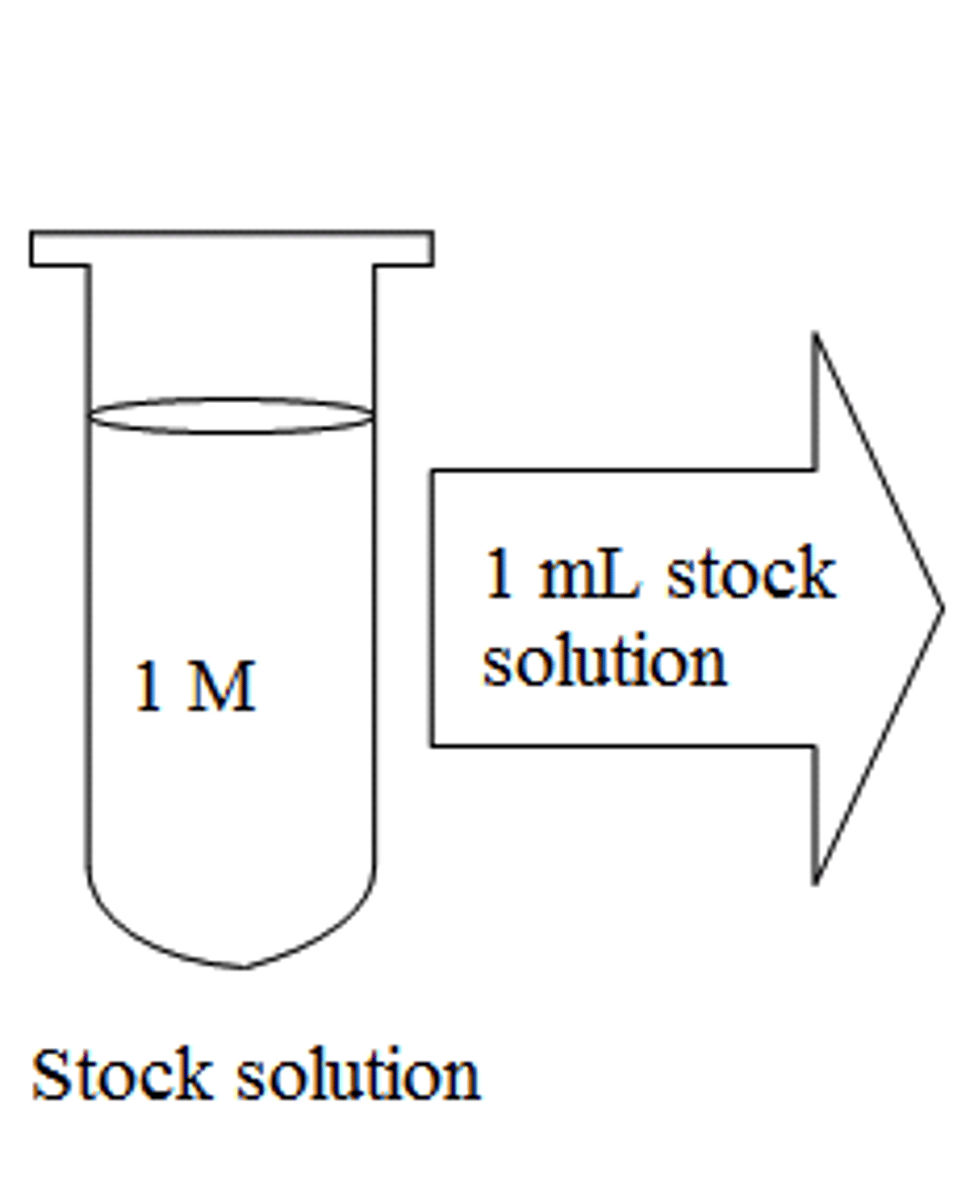
Stock solution
A stock solution is a concentrated solution that will be diluted to some lower concentration for actual use. Stock solutions are used to save preparation time, conserve materials, reduce storage space, and improve the accuracy with which working lower concentration solutions are prepared.
v/v and w/v
This means the percent by weight of solute in the total volume of solution. Normally used where the solute is a solid. For example, a 10% w/v sodium chloride solution would be prepared by dissolving 10 grams of sodium chloride in a solvent (again, say, water) then making up to a final solution volume of 100ml