Lecture 3 Pharmacodynamics and Terminology
Clearance
rate of elimination of drug in an hour
1st order elimination kinetics
elimination proportionate to the drug serum concentration
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Clearance
rate of elimination of drug in an hour
1st order elimination kinetics
elimination proportionate to the drug serum concentration
1st order elimination kinetics calculated
Cl = elimination/peak plasma concentration
total amount of urine voided in an hour : peak plasma concentration of drug
Zero order elimination kinetics
rate of elimination or clearance is constant - no matter the dosage
e. ethanol and aspirin
alcohol clearance
1-2 drinks = 20-30mg/dL
clearance rate = 20mg/dL/hr
ETOH poisoning Er admmission average =>295 mg/dL
0.08 = 80mg
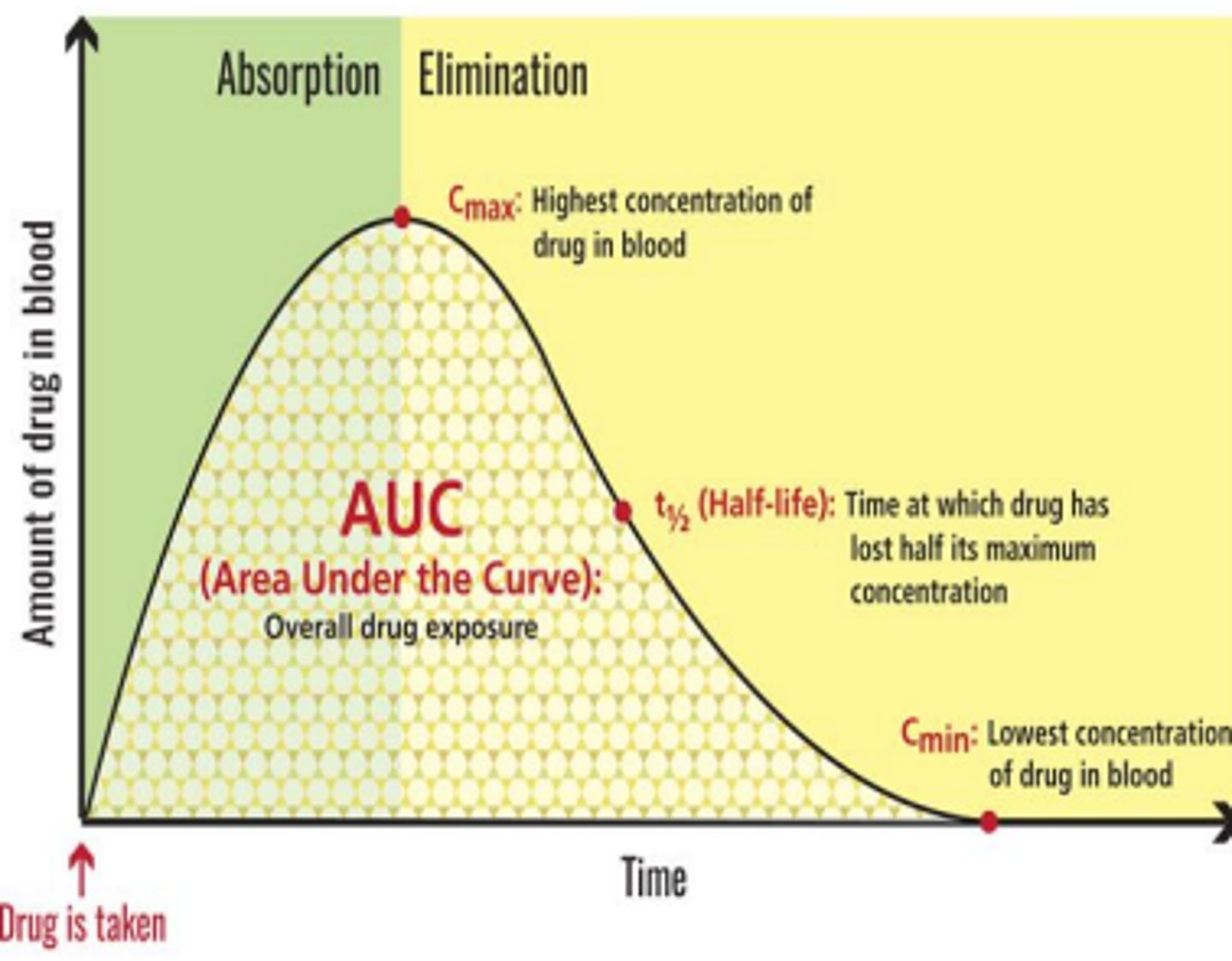
Half-life (t1/2)
time required for Cmax drug plasma concentration to decrease by one half (50%)
Used to estimate frequency of administration
Cmax
the maximum concentration of drug dose
drug circulation
circulating drug is continuously bio transformed for excretion - if 'unchanged' = biotransformation not required for excretion
half-life calculation
calculated & known per each drug. broad guideline to estimate frequency of adminitration
4x t1/2 = 90% of drug cleared
pharmacodynamics
once a drug is distributes - where does it cause the therapeutic effect
drug-receptor binding is:
saturable, dynamic (increases or suppresses existing processes) and reversible
bind directly to/in bacteria/viruses
some drugs like anti-infective. They present antigen that WBC recognize and try to destroy
they have different mechanisms of action = some interrupt bacteria cell wall, protein synthesis
most drugs bind protein structures to =
receptors
receptor affinity
strength of binding or length of binding. faster acting drugs are very potent but may not have high affinity
characteristics of receptor affinity
is specific, saturable, reversible.
What is drug efficacy?
The degree to which a drug induces maximum therapeutic effect.
What is an example of a drug used for motion sickness nausea?
Gravol
What is an example of a drug used for chemotherapy-induced nausea?
Ondansetron
What does drug efficacy depend on?
The cause of the condition being treated. what works where, best
Potency of a drug
= strength
how much of the drug is required = amount
ex. drug A 20mg & drug B 10 mg = Drug B potency is higher
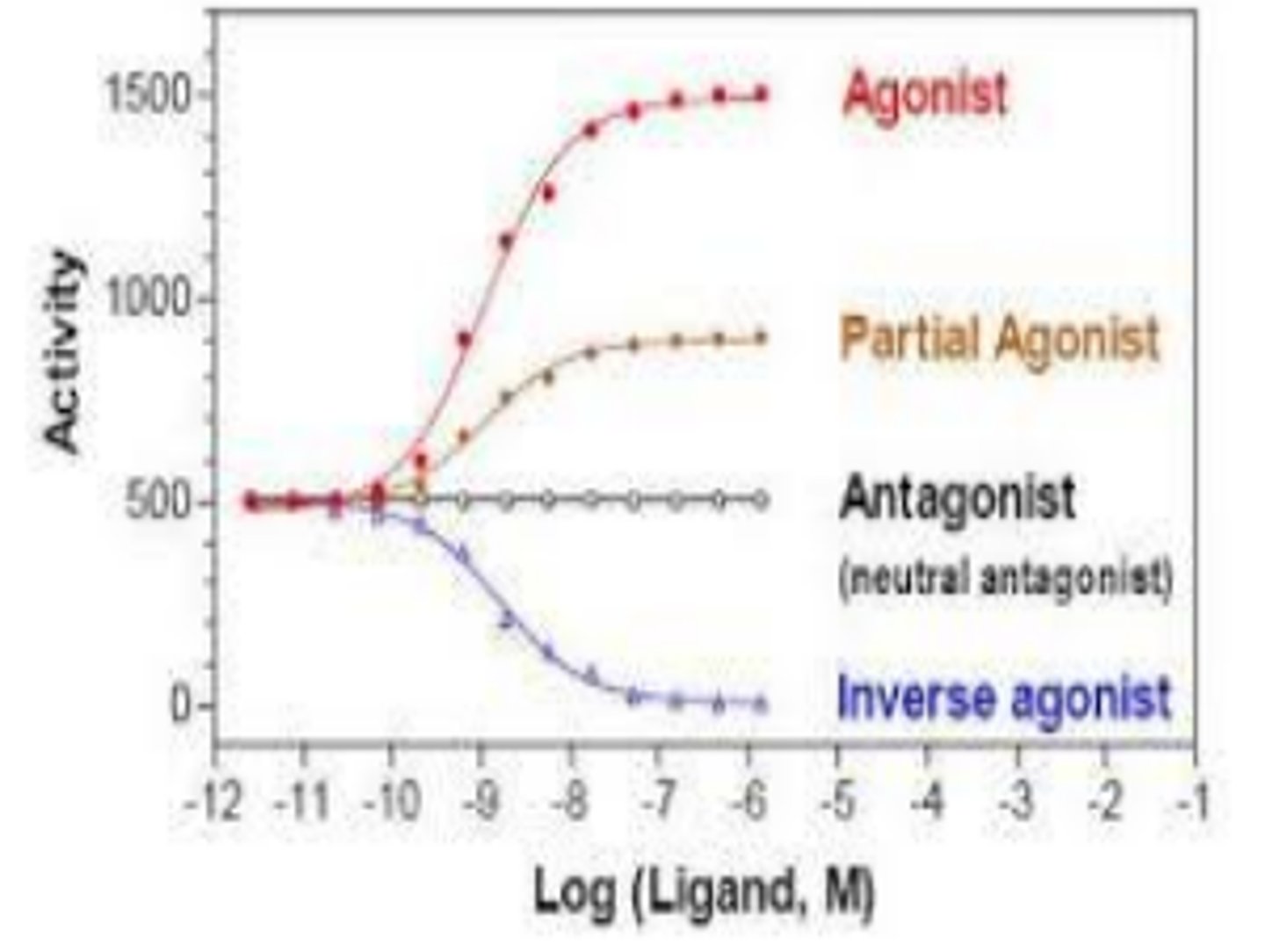
agonists
drug mimics the endogenous substance (support normal activity). it binds to receptors readily and produces good effects
ex. morphine
primary (full) agonist
extensively and successfully binds to existing receptor
What is a partial agonist?
A drug that produces a smaller maximum response even when all receptors are occupied.
What is low efficacy in pharmacology?
It refers to a drug that has a lower maximum effect compared to full agonists.
Give an example of a partial agonist.
Buprenorphine
What is a common full agonist that is often compared to buprenorphine?
Morphine
what happens when full agonist and partial agonist meet?
in the presence of a full agonist, a partial agonist acts like an antagonist. they compete with each other for binding
when do you need a partial response
when you ween patients off of pain meds
inverse agonist
binds to receptor but induces the opposite effect of the naturally binding substance.
ex. caffeine
how is caffeine an inverse agonist
it binds to receptors for adenosine (naturally calming substance) - caffeine doesn't stimulate SNS it just stops PSNS
antagonists
drug blocks the receptor site to prevent endogenous or endogenous-like substance form binding. no effect other than to block other substances from binding
no efficacy at cellular level
example of antagonist
naloxone (Narcan) - has higher affinity to receptors than opioids, has shorter half-life so you need more dosages of Narcan to meet level of drug you want to displace
6 Major receptor types
1. G-protein (GPCR)
2. Ion channels
3. Nuclear receptors
4 & 5. Enzyme types
6. Non-enzyme 'JAK-STAT'
G-protein
most common
g-protein binding elicits a change.
may be alpha, beta, alpha1 etc. - can selective or nonselective drugs
triggers intracellular messenger - opens or closes channel
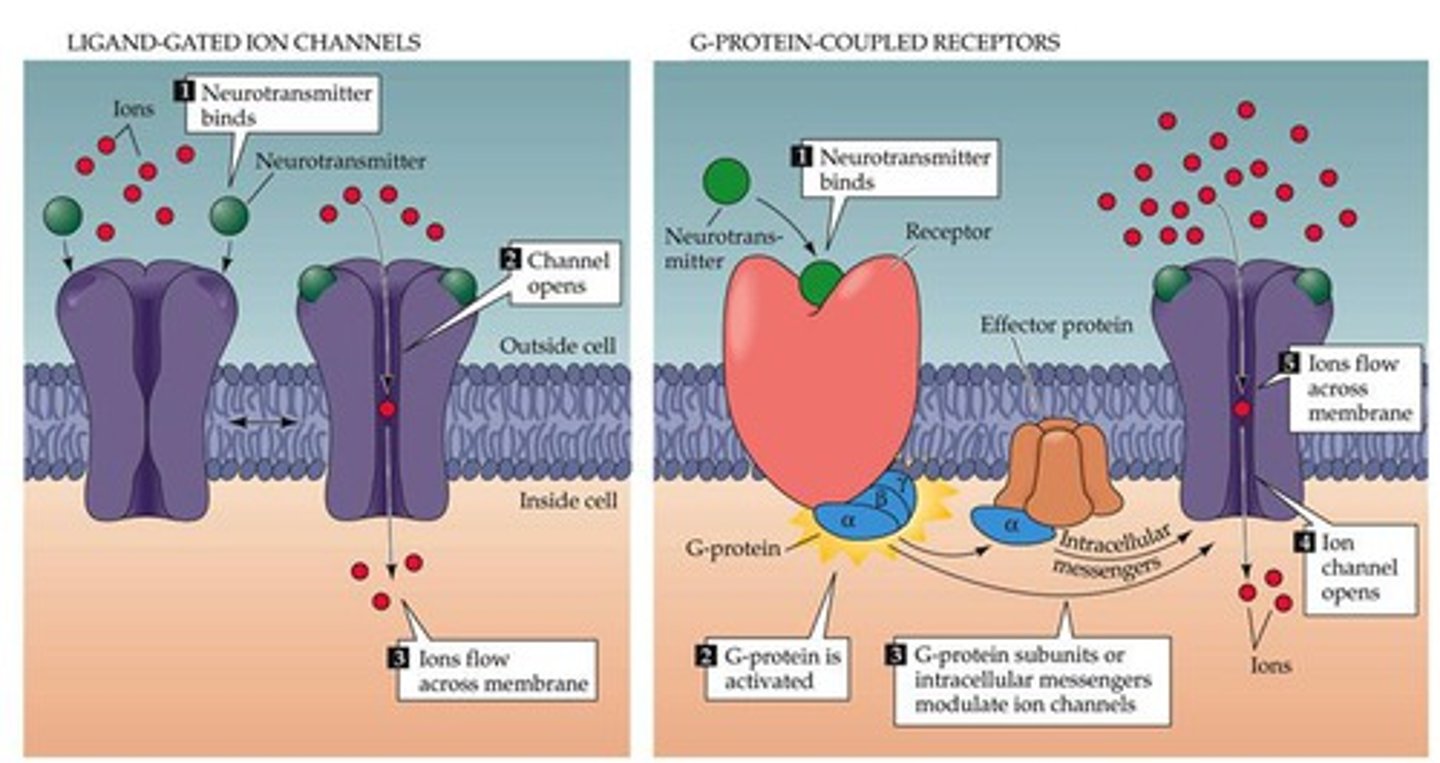
g-protein example
epinephrine - stimulates G-protein & second messenger system =sympathomimetic stimulation
ion channels
electrolyte movement. - most bind to voltage-gated channels.
ex. lidocaine (local anesthesia) - closes ion channels = no action potential = no cellular depolarization = no pain transduction
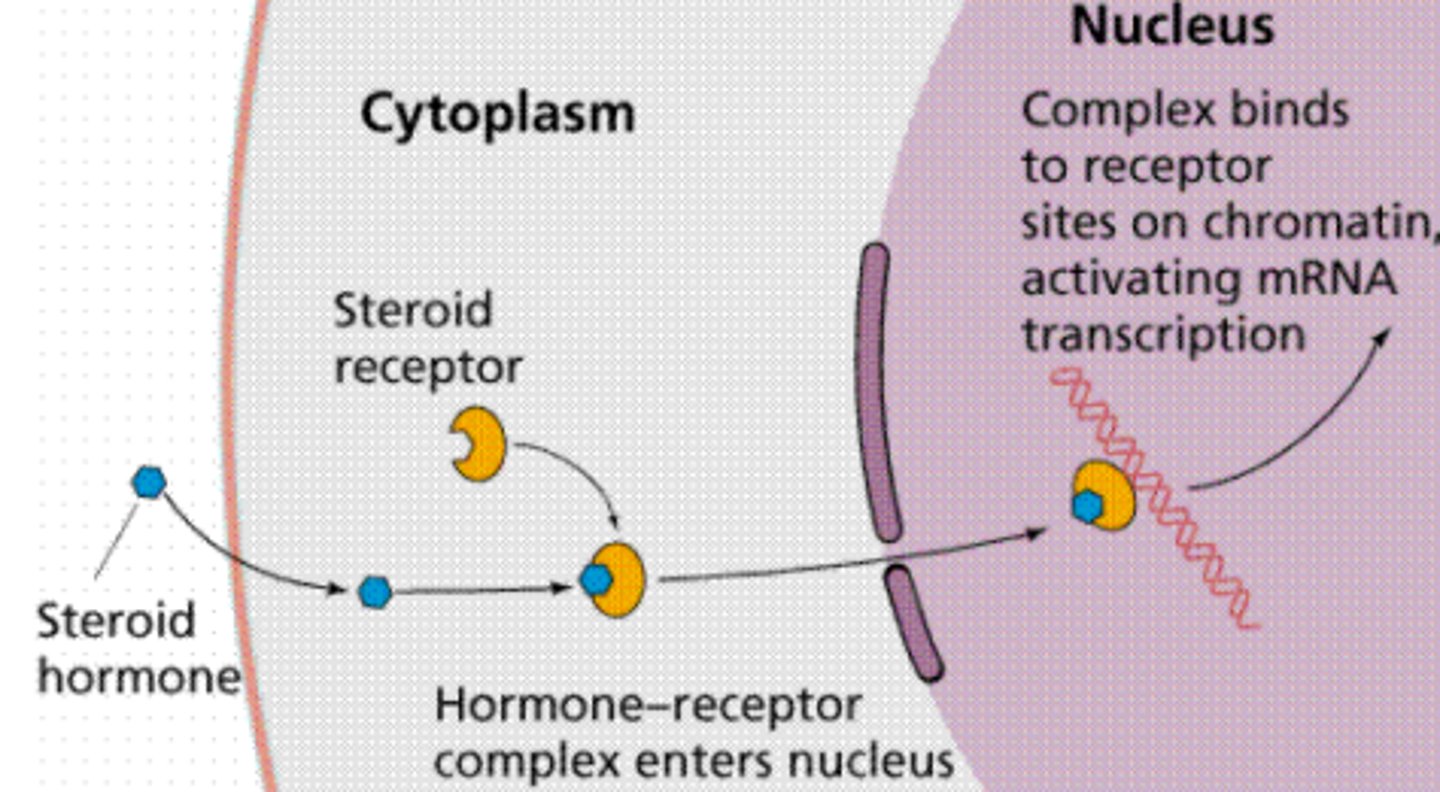
nuclear receptors
ex. hormones
aka. steroid receptors
most bind in cytoplasm = change cell via DNA.
Drug need to be lipophillic to enter plasma membrane
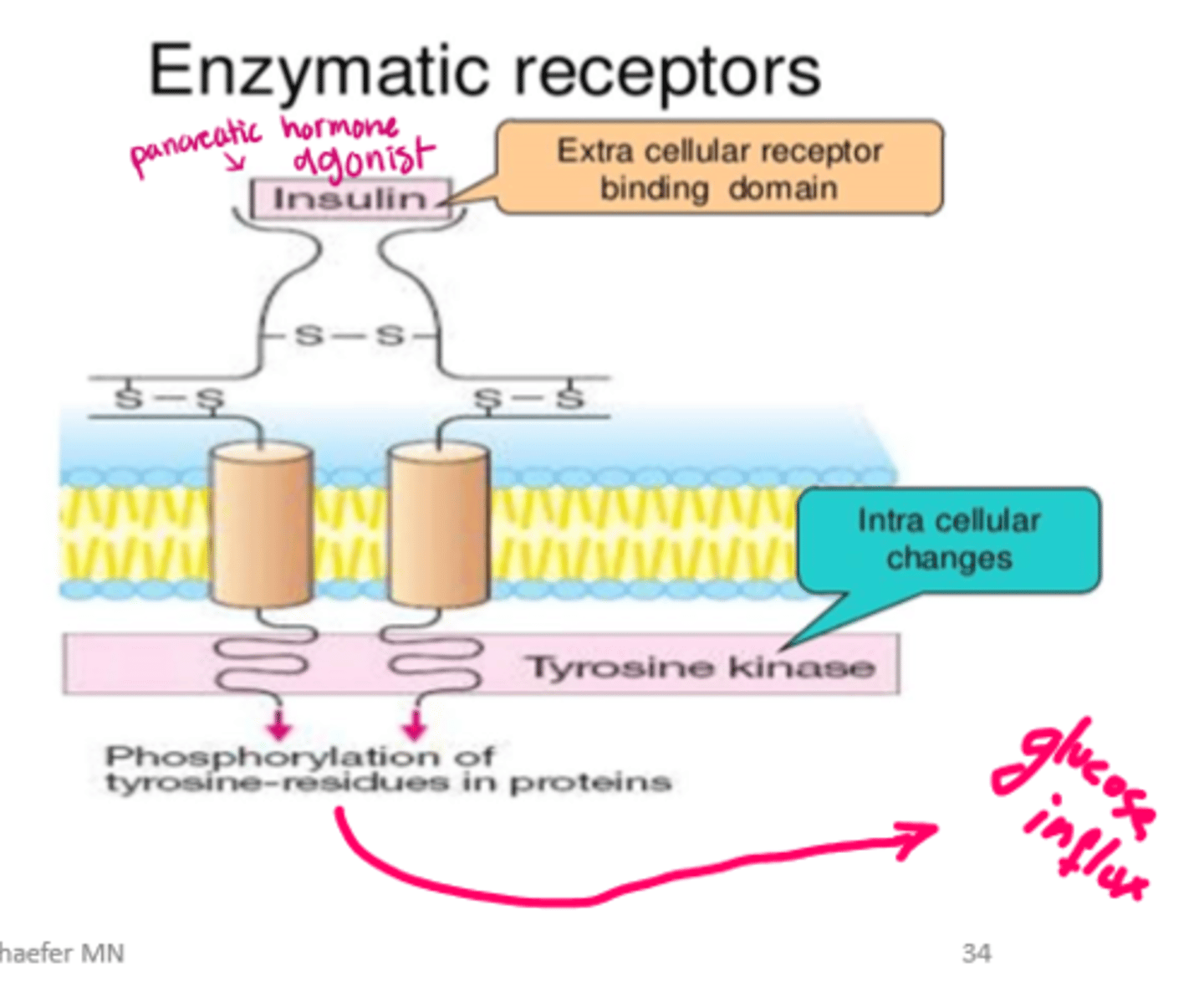
enzyme binding 2 types
intracellular enzymes
transmembrane enzymes
example of transmembrane enzymes
insulin => cell membrane receptors = stimulate transmembrane enzyme: tyrosine kinase => activate cellular glucose uptake
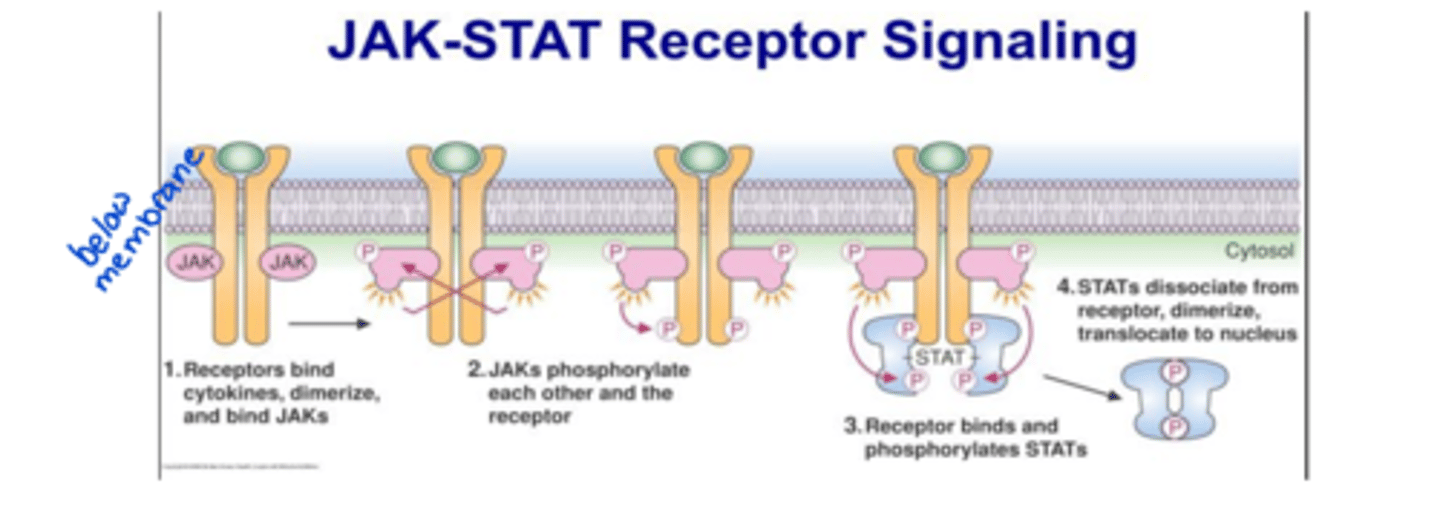
non-enzyme transmembrane
eg. JAK-STAT receptors (used by interferons)
STAT attaches and creates change
What is drug tolerance?
The need for more of a drug to achieve the same effect due to receptor desensitization or decreased number of viable receptors.
What is receptor desensitization?
A process where receptors become less responsive to a substance.
What are some examples of addictive substances that can cause drug tolerance?
Opiates, benzodiazepines, and alcohol.
drug resistance
many variations of kinetic alterations
Ex. increased drug metabolism = drug metabolized at a faster rate, therefore less bioavailable therefore less effective.