part 24: PHASE DIAGRAM
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Phase Diagram
Represents the states of matter that exists as temperature and pressure varied
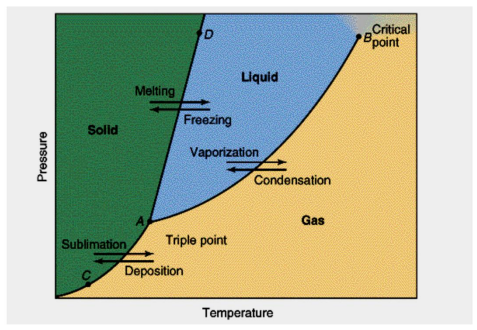
Melting curve/Freezing curve
AD
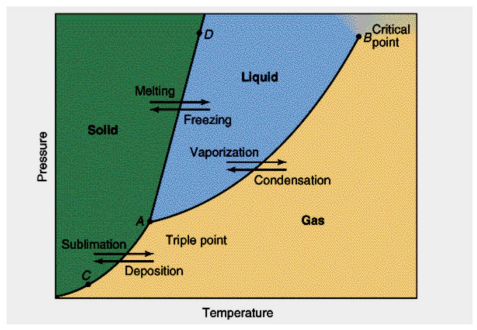
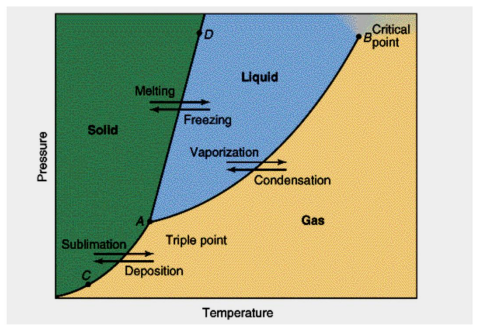
Sublimation/Deposition curve
AC
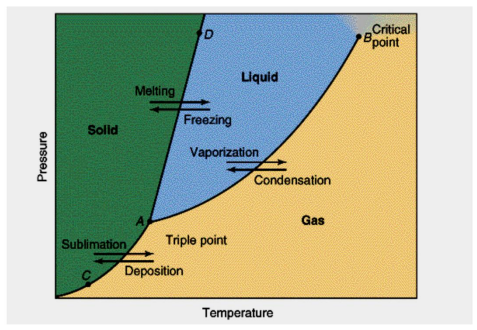
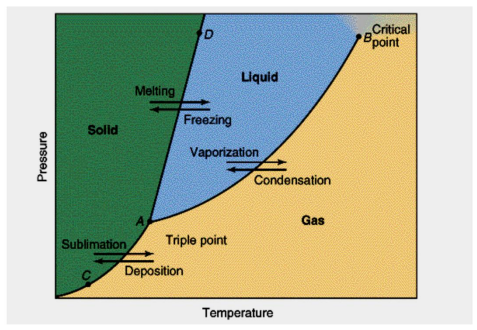
Vaporization/Condensation curve
AB
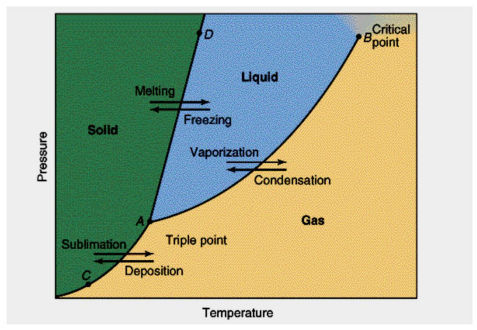
Critical Temperature
Temp beyond w/c liquid will no longer exist
Critical Fluid
System that will exist beyond critical temperature and critical pressure
Used in decaffeination of coffee
Partly liquid and gas in character
Gibbs Phase Rule
Phase Rule is also known as?
Phase Rule
Is a relationship for determining the least number of independent variables (e.g., temperature, pressure, density, and concentration) that can be changed without changing the equilibrium state of the system
The number of variables that may be changed independently without causing the appearance of a new phase or disappearance of an existing phase
F=C-P +2
Wheras:
F: the number of degrees of freedom of the system (number of independent variables (e.g. temperature, pressure, and concentration) that may affect the phase equilibrium)
C: number of components
P: number of phases
Formula of Phase Rule
2
Determine the degrees of freedom of a system containing either ice, water or water vapour
A. 1
B. 2
C. 3
D. 0
1
What is the degree of freedom for a system containing a water in equilibrium with its vapour A. 1
B. 2
C. 3
D. 0
0
Assuming that we have system wherein ice-water-water vapour are in equilibrium. What will be the degrees of freedom of the system.
A. 1
B. 2
C. 3
D. 0
1 phase
Number of phases of Gas, Liquid, or Solid system
2
Degrees of Freedom of Gas, Liquid, or Solid system
Bivariant
2 Degrees of Freedom
2 phases
Number of phases of Gas-Liquid, Liquid-Solid or Gas-Solid system
1
Degrees of Freedom of Gas-Liquid, Liquid-Solid or Gas-Solid system
Univariant
1 Degree of Freedom
3 phases
Number of phases of Gas-Liquid-Solid system
0
Degrees of Freedom of Gas-Liquid-Solid system
Invariant
0 Degrees of Freedom
Increases
As the number of components INCREASES, the degrees of freedom _______________
Decreases
As the number of phases INCREASES, the degrees of freedom _______________