Lecture 3: Radial wavefunctions & energy levels of hydrogen
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
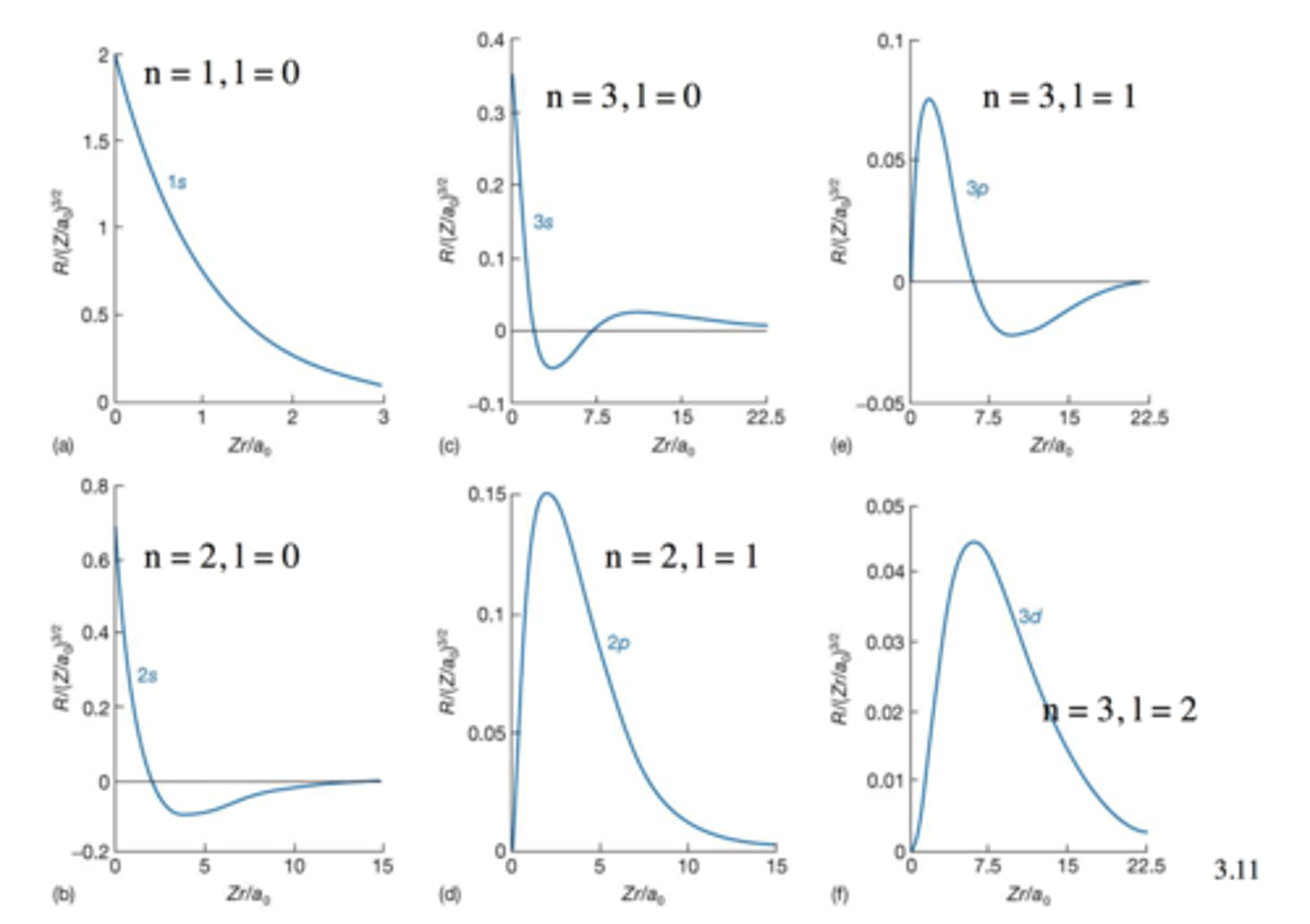
Radial Node
- occur where the radial component of the wave function passes through zero
- total number of radial nodes for any s orbital (ns) equal to n-1
np has n - 2 radial nodes
nd has n - 3 radial nodes
nf has n - 4 radial nodes
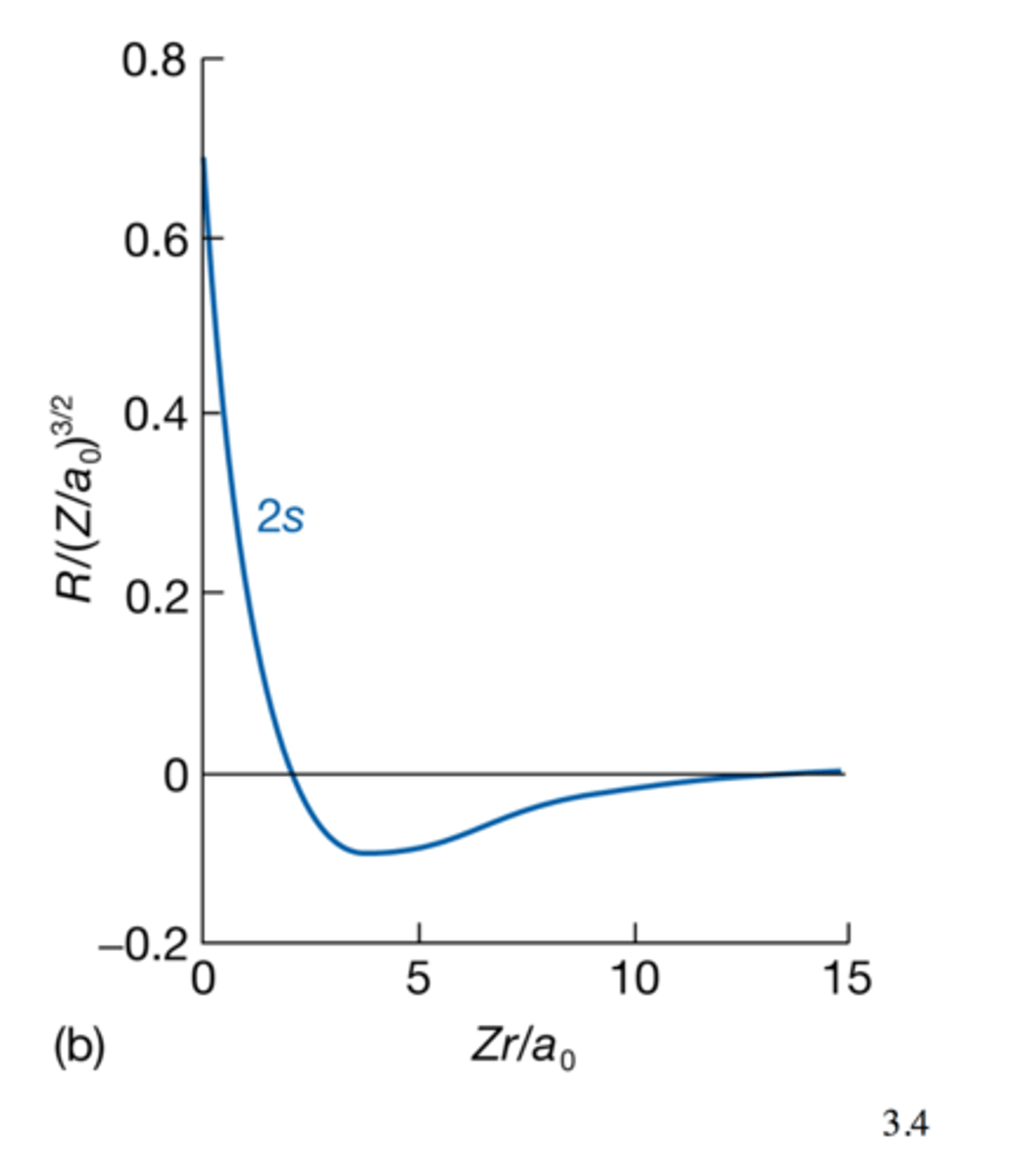
2s Wavefunction
- n=2 ,l=0, ml =0, wavefunction known as 2s
- ψ is non zero at nucleus
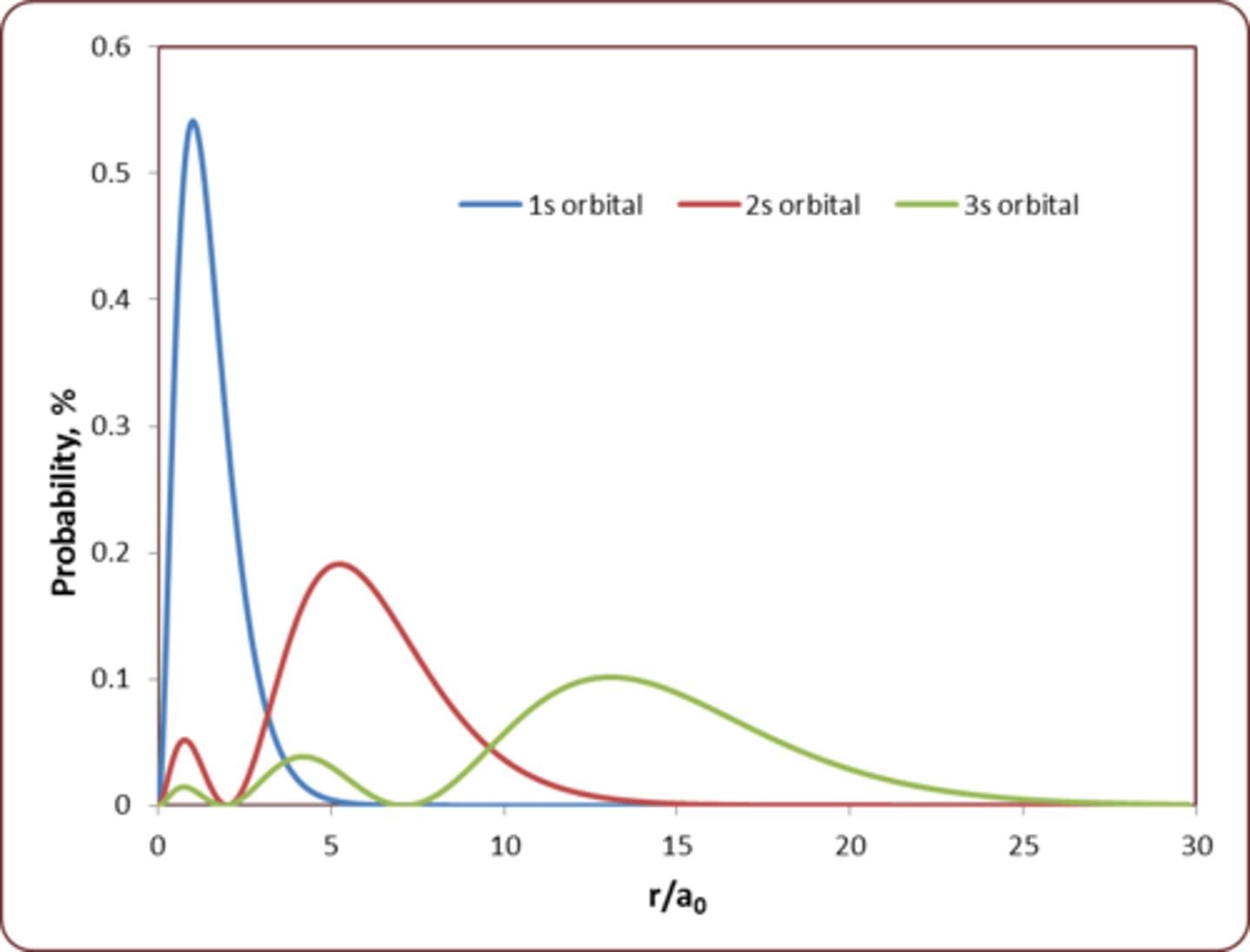
passes through zero, 1 node at r = 2
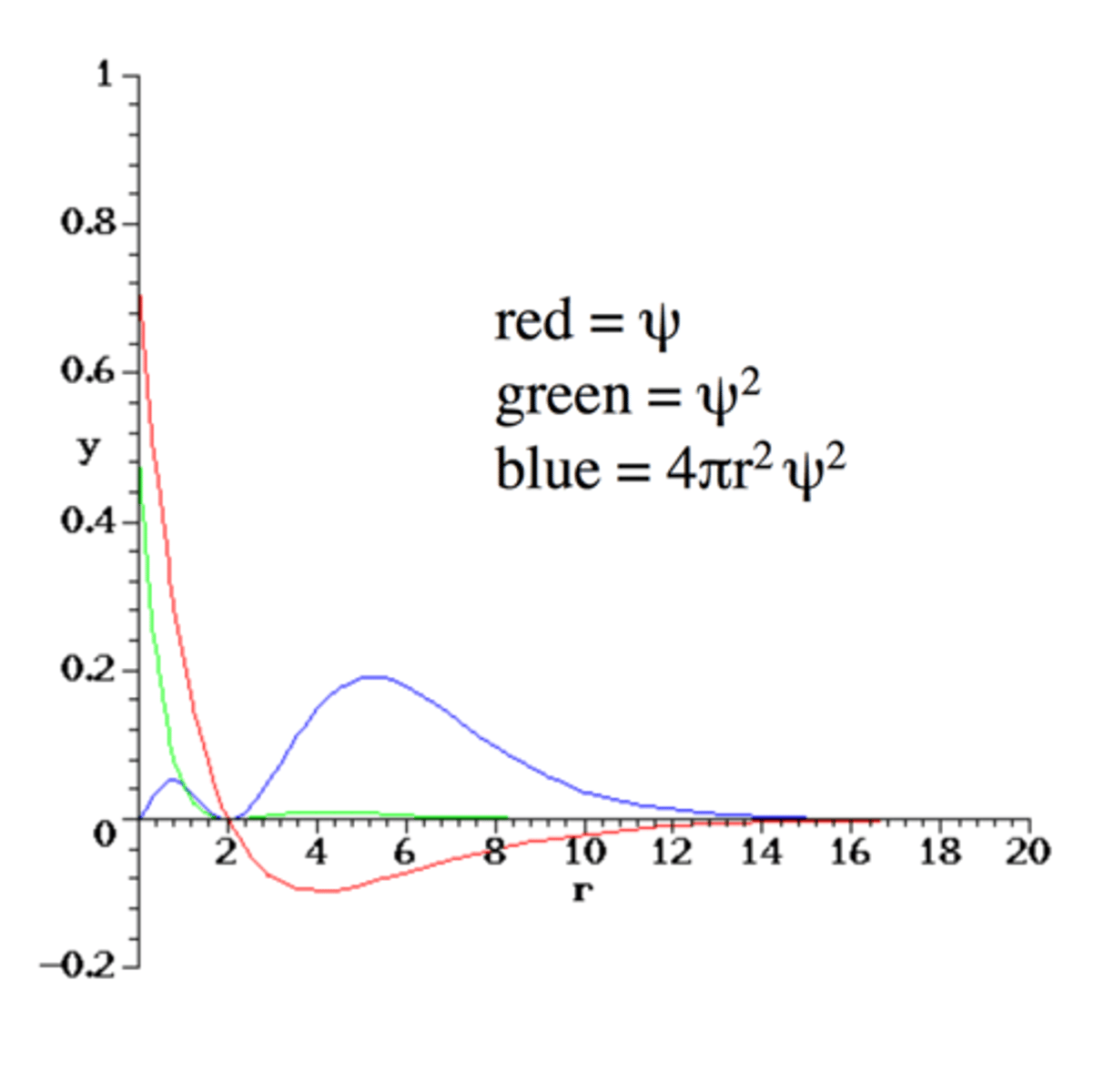
Radial Distribution for 2s
- Two local maxima in electron
density / probability
- Most probable radius for the electron in a H atom when it is in the 2s orbital is at about 5 a₀
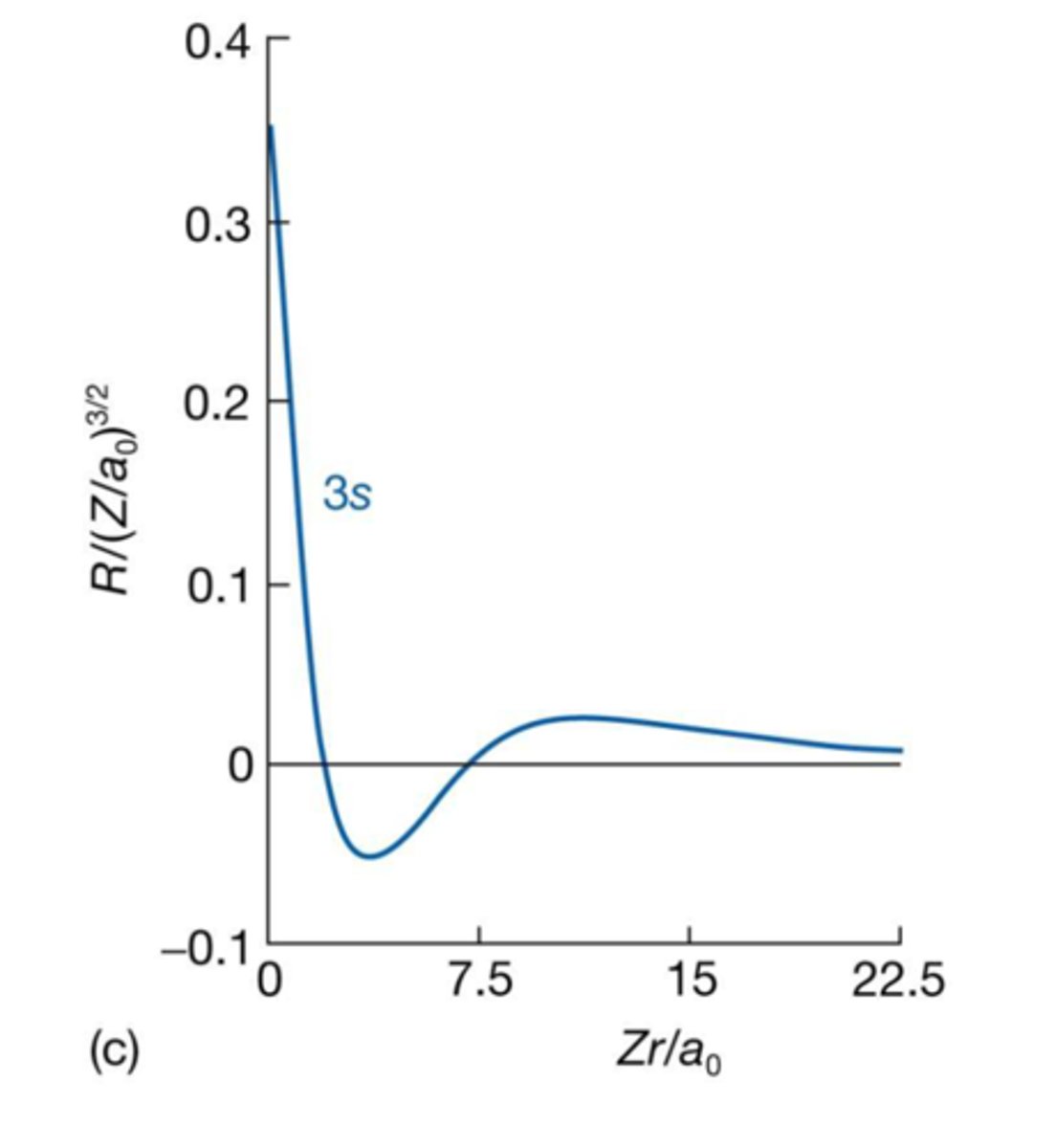
3s Wavefunction
- n=3, l=0, ml =0, wave function known as 3s
- ψ is non zero at the nucleus, 2 nodes
Radial Distribution for 3s (compared to 1s and 2s)
- 3s has 3 local maxima
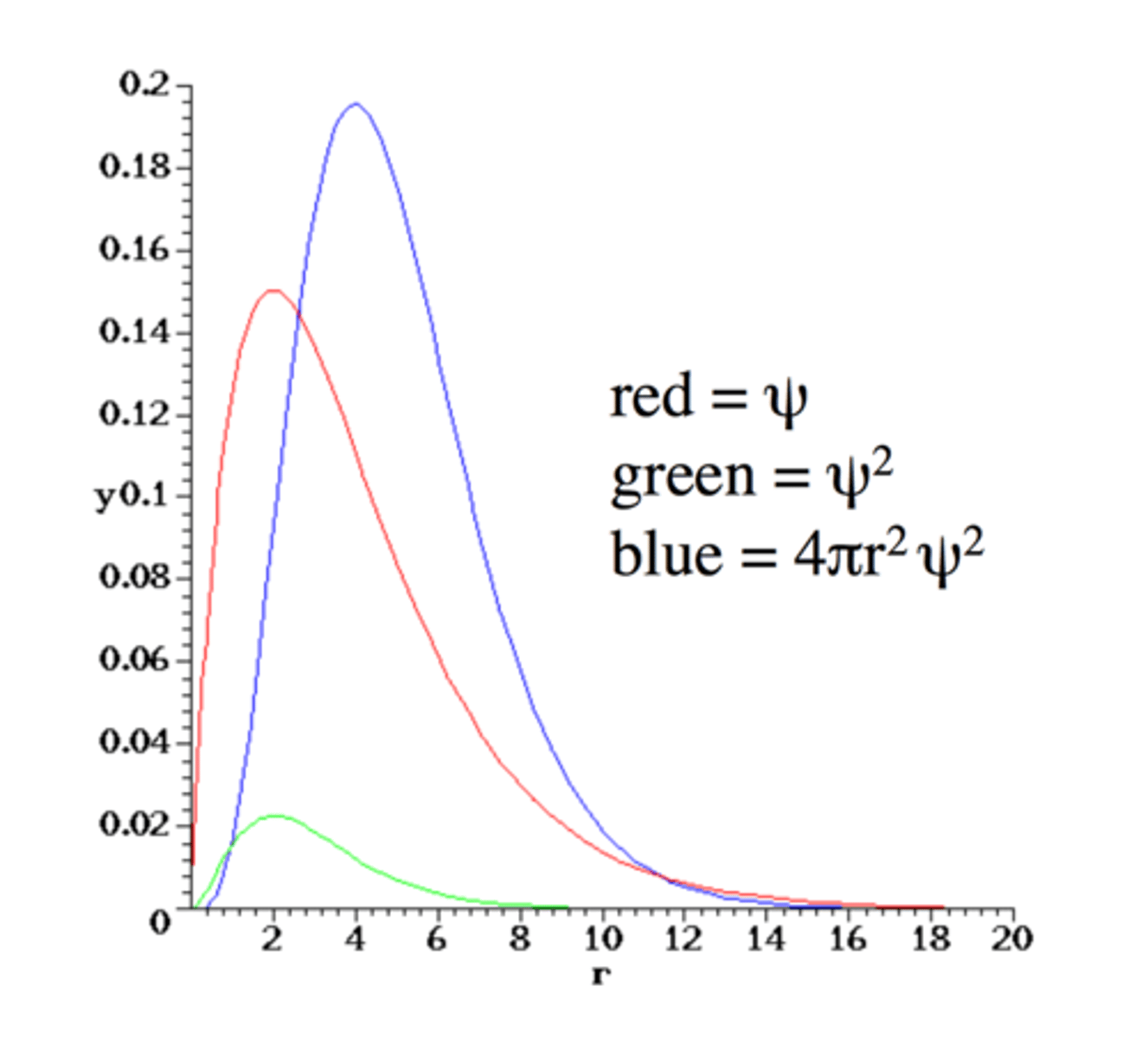
2p Wavefunction
- n=2, l=1, ml = -1, 0, +1
- Maximum for radial distribution is at 4 a₀
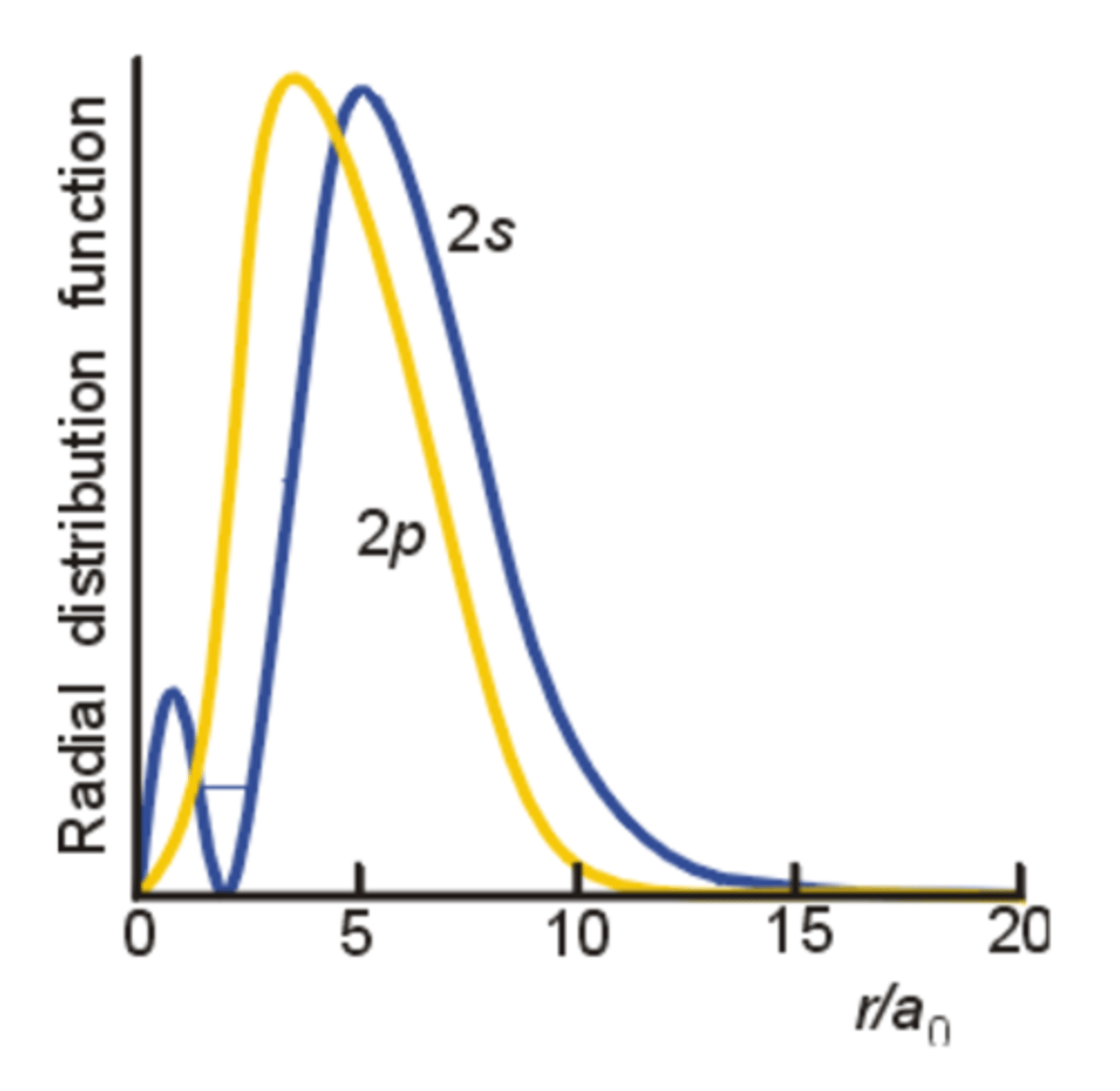
Comparison between 2p wavefunction and 2s
- 2p is on average closer to the nucleus, it maximum is nearer
- The 2s has a high probability of being very close to the nucleus - due to the small inner maximum
- Penetration is the potential for the presence of an electron inside shells of other electrons
- A 2p electron does not penetrate so effectively through the core (filled inner shells of electrons) because its wave function goes to zero at the nucleus. As a result, it is more fully shielded from the nucleus by core electrons
- This means in a many-electron atom, a 2s has a lower energy and will be occupied before the 2p
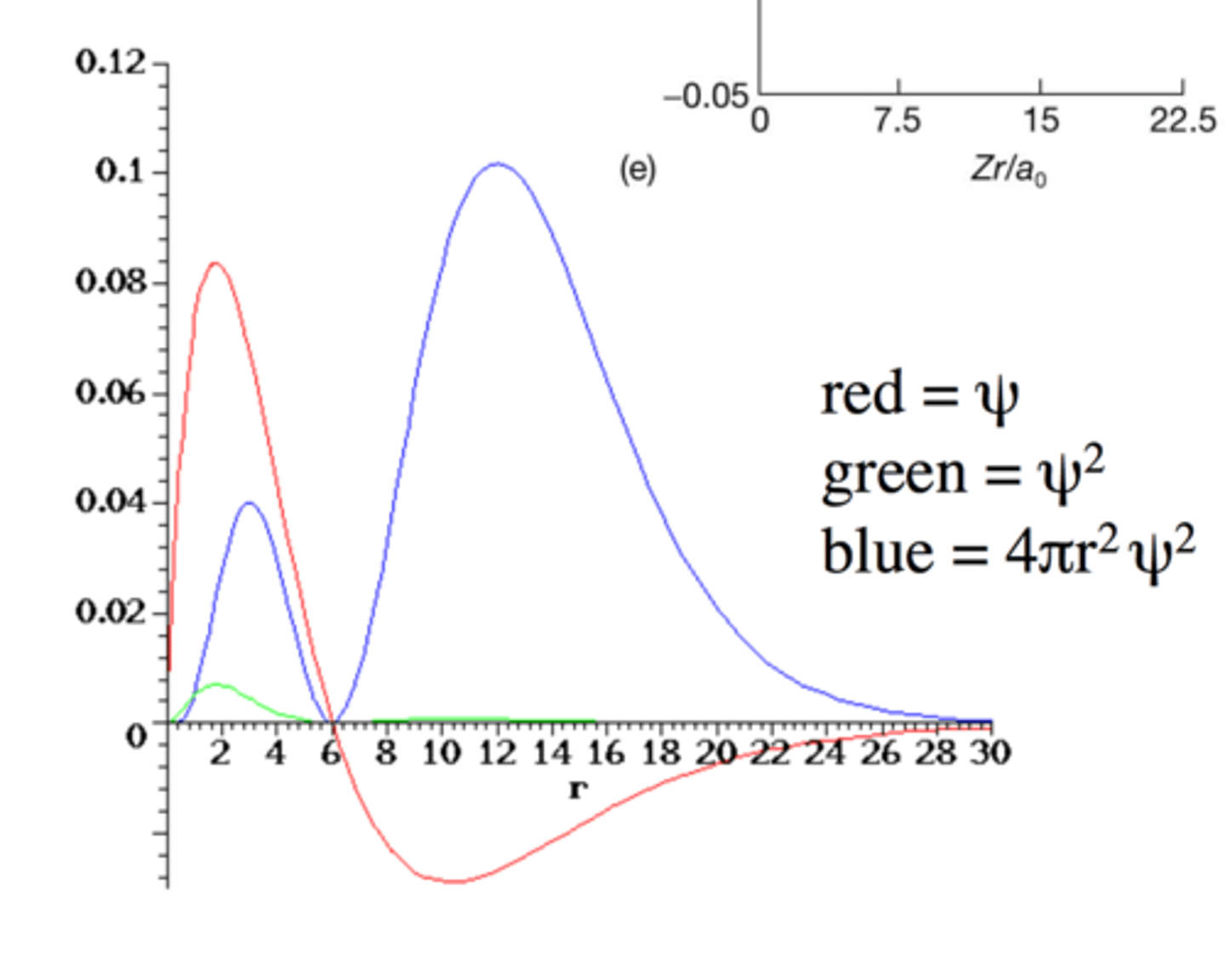
3p Wavefunction
- n=3, l=1, ml = -1, 0, +1
- 1 radial node
- Two maxima for radial distribution curve
3d Wavefunction
- n=3, l=2, m= -2, -1, 0, +1, +2
- no radial nodes (nd has n-3 radial nodes)
- Maxima in radial distribution curve is at 9 a₀
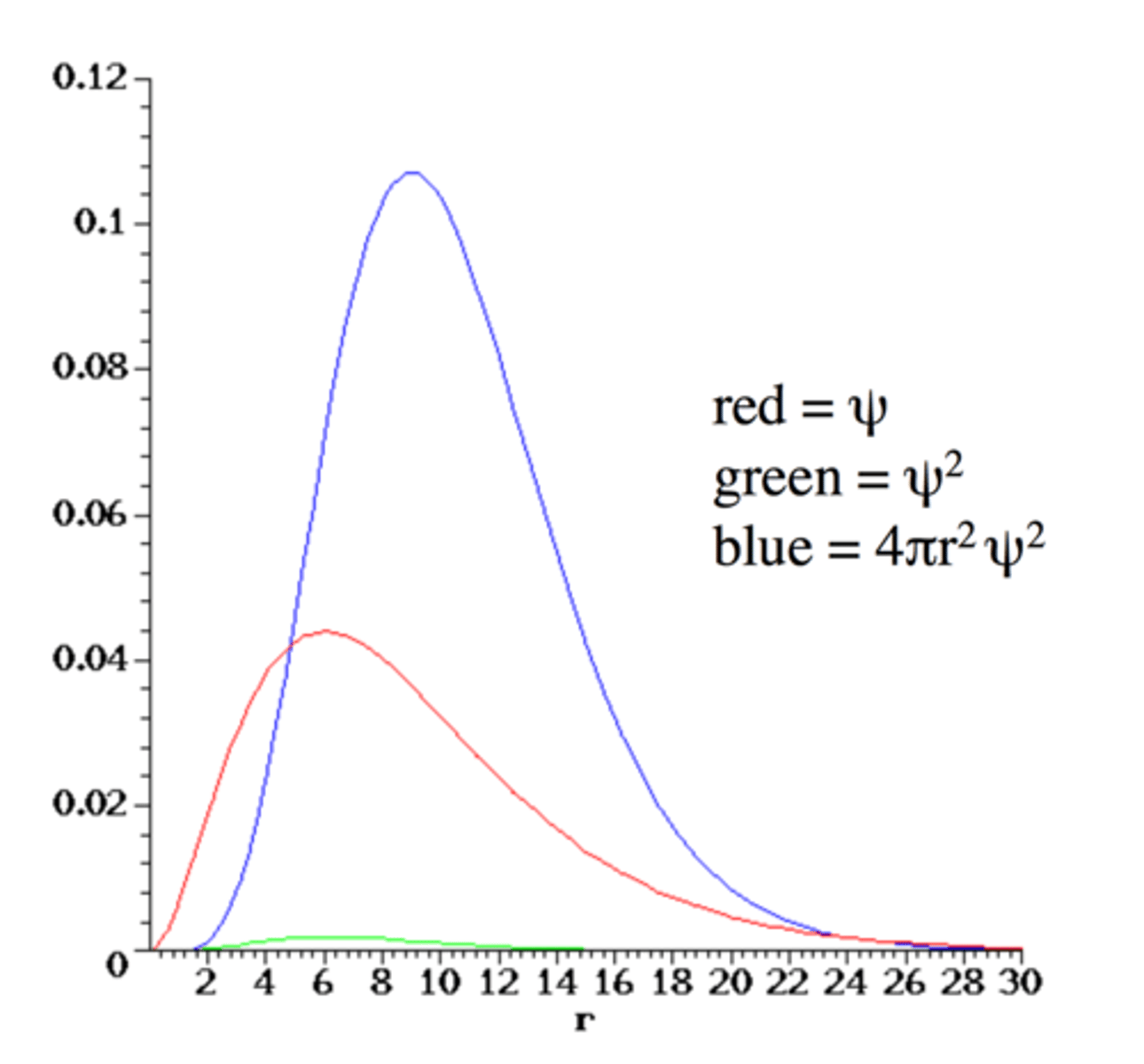
All relevant wavefunction graphs