Alcohols
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Alcohols
Functional group - OH
General formula - CnH2n+1OH
Physical properties
they have a higher MPT and BPT than their corresponding alkane
soluble in water
alcohols can form hydrogen bonds
Combustion
observation = clean blue/pale yellow flame
C2H5OH + 302 → 2C02 + 3H20
Halogenation
Chlorination:
i) Add PCl5 to alcohol and mix at room temperature in a fume hood
e.g. CH3CH2OH + PCl5 → CH3CH2Cl + POCl3 + HCl (steamy white fumes that turn damp blue litmus paper red (acidic)
ii) conc. HCl - reflux then distil
Bromination:
KBr + 50% H2S04 (makes HBr so 50% avoids HBr oxidising to bromine) and heat under reflux
Iodination:
Damp red phosphorous + iodine (moisture allows solids to come into contact and react)
2P + 3i2 → 2Pi3
Oxidation
alcohols are oxidised by K2Cr2O7 / H2SO4
K2Cr2O7 is an oxidising agent so is reduced itself
Cr (VI) = orange
Cr(III) = green
Primary OH:
1 OH → aldehyde = distillation
1 OH → COOH = heat under reflux
Secondary OH:
2 OH → ketone (cannot oxidise further) = reflux
Tertiary OH:
no reaction
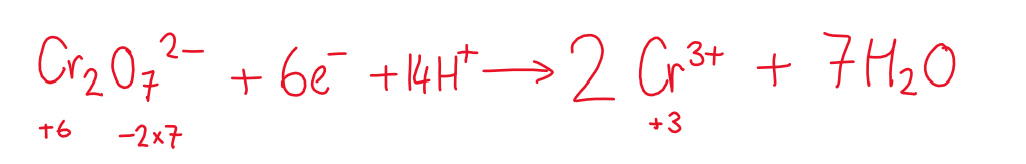
Elimination
Alcohol → alkene + water
method 1 - pass the alcohol over a hot aluminium oxide catalyst
method 2 - mix the alcohol with H3P03 and warm
Testing for alcohols
testing for OH → add PCl5
Positive result = steamy white fumes which turns damp blue litmus red
But this could be OH in COOH
Testing for 3 OH → add acidified K2Cr2O7 and warm
Positive result = stays orange
If 1 or 2, turns green
Testing for 1 → oxidise first and distil off product, then add benedict’s solution
Positive result → blue solution changes to/forms brick red ppt. = aldehyde
Negative result → stays blue = ketone