PPFA6 - driver of a reactions
1/18
Earn XP
Description and Tags
gibbs free energy ,third law significance
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
what happens to the entropy of a perfect crystalline substances as they approach zero?
when the temperature approaches absolute zero ( T=0 k )
at 0 K ,atoms are perfectly ordered = no randomness- S=0
What make it absolute?
-before the third law entropy was relative ,could only compare changes in entropy ∆s
the third law gives us a reference point :
-at absolute zero (0K) ,a perfectly crystalline substance has zero entropy ,s=0
What is gibbs free energy function?
second law - a process spontaneous if the total entropy of a system plus surroundings increases
use gibbs free energy to predict spontaneity and equilibrium based only on system properties
ΔS_total = ΔS_system + ΔS_surroundings ≥ 0
what is gibbs free energy used to describe ?
Gibbs free energy (G) is used to describe energy changes at constant pressure and temperature, defined as:
G = H − TS
(H = enthalpy, T = temperature, S = entropy)
The change in free energy is:
ΔG = ΔH − TΔS
What is the gibbs free energy at constant temperature and pressure and what do they mean ?
ΔG < 0 → the process is spontaneous
ΔG = 0 → the system is at equilibrium
ΔG > 0 → Process non-spontaneous
What does free energy reflect?
Free energy reflects the system's capacity to do useful (non-expansion) work:
ΔG = maximum non-expansion work (wₘₐₓ)
In real systems, this work can take forms such as:
Electrical work (e.g., in electrochemical cells)
Biological energy storage (e.g., ATP molecules)
What is the gibbs free energy criteria for spontaneity ?
The sign of Gibbs free energy change (ΔG) determines whether a process is
spontaneous, non-spontaneous, or at equilibrium (at constant temperature and
pressure)
From the Gibbs equation:
ΔG = ΔH − TΔS
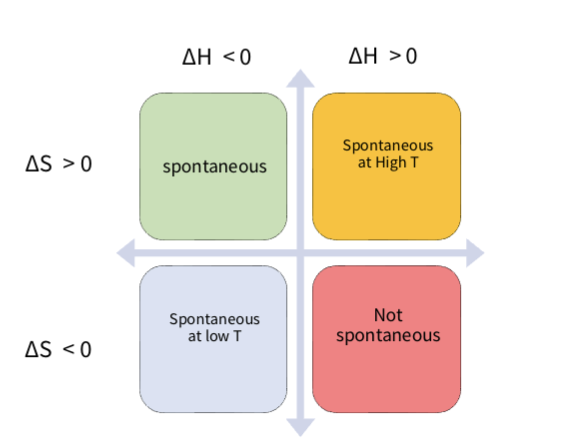
gibbs free energy spontaneous processes summary
The sign of Gibbs free energy change (ΔG) determines whether a process is spontaneous,
non-spontaneous, or at equilibrium (at constant temperature and pressure)
From the Gibbs equation:
ΔG = ΔH − TΔS
▪ If ΔH is negative (exothermic) and ΔS positive → process always
spontaneous
▪ If both ΔH and ΔS are negative → spontaneity depends on T (low T)
▪ If both are positive → spontaneity also T dependent (high T)
▪ If ΔH positive and ΔS negative → never spontaneous
describe what happens to entropy and gibbs in dissolution
-Dissolution of salts in water — entropy increases due to random distribution of solute
-molecules, making ΔG negative.
-the Replacement of crystal molecules with solvent molecules during dissolution
what is the importance of third law in pharmacy ?
helps predict spontaneity
stability of drug molecules and formulations
phase changess during drug manufacturing and storage
Give some Examples of gibbs free energy in pharmaceutical science
predicting solubility
determining feasibility of formulation reactions
understanding dissolution processed of solid dosage forms
evaluating temperature dependence of drug stability
what are the differences between enthalpy driven reactions and enthalpy driven reactions ?
Some processes are spontaneous due to favorable enthalpy (ΔH < 0), like combustion
Others are spontaneous due to favorable entropy (ΔS > 0), even if they require energy
input (ΔH > 0), like dissolution of salts
Phase transitions (e.g., melting, vaporization) are often entropy-driven at higher
temperatures
Entropy effects dominate at high T
Enthalpy effects dominate at low T
what is the hydrophobic effect and gibbs free energy ?
Amphipathic molecules (like phospholipids) that have both hydrophobic tails and hydrophilic heads
Molecules arrange into layers or micelles due to the hydrophobic effect
• In water, nonpolar regions cluster together to minimize unfavourable interactions
create micelles- In water – useful to enhance the solubility of the drug in water
Some molecules ( polymer),phospholipids ) – when places in water some like water some want to escape from the water as they are less water soluble - they go into a more favourable orientation – driven by Gibbs free energy more energetically stable
what is the link for gibbs free energy and amiphpatihc molecules?
Gibbs Free Energy Link:
-Water forms 'cages' around hydrophobic molecules → low entropy (unfavourable, +ΔG)
-Clustering reduces cages, frees water → higher entropy (favourable, -ΔG)
-Entropy-driven process → spontaneous (ΔG < 0)
give an example of the hydrophobic effect in pharmacy
Drug encapsulation in liposomes/micelles improves solubility of hydrophobic drugs (e.g., Amphotericin B in liposomal formulations)
•For Severe and systemic infection
•Liposomal amphotericin B is generally less nephrotoxic (damaging to the kidneys) than conventional amphotericin B, making it an option for patients with impaired renal function
what is the biological relevance of the hydrophobic effect?
Hydrophobic interactions drive protein folding → functional structure
Low temperatures weaken interactions → protein unfolding (cold denaturation)
insulin storage example:
It’s really hot outside today. I’ll be out shopping for a while before going home. Do I really
need to keep the insulin cold? Won’t it be fine in my handbag for a few hours?
-Insulin is a protein and can lose its structure when exposed to heat
Keeping insulin in the fridge (2–8 °C) slows down degradation reactions
degrades faster because increase in entropy so T∆s increases
what affect can freezing have on insulin ?
At very low temperatures:
Hydrophobic interactions become weaker (water molecules around nonpolar groups
become more ordered).
This can also destabilize the folded structure → cold denaturation
Thermodynamics link:
Folding is governed by Gibbs free energy (ΔG = ΔH – TΔS)
At high T → entropy dominates (TΔS large), driving unfolding
At very low T → enthalpy changes (weaker hydrophobic effect) can outweigh entropy,
again destabilizing the protein
summary card
ΔG = ΔH − TΔS links enthalpy, entropy, and spontaneity
❑ Negative ΔG → spontaneous; positive ΔG → non-spontaneous
❑ Spontaneous changes are irreversible