Chemistry IB HL: Atomic Structure
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Mass number
The number of protons plus the number of neutrons
Atomic number
The number of protons in the nucleus
Isotopes
Atoms of the same element with the same number of protons (atomic number) but a different number of neutrons (mass number)
Properties of isotopes
Same chemical properties, different physical properties (e.g. boiling/melting point)
Relative atomic mass (Ar)
Ratio of the average mass of the atom to the unified atomic mass unit
Formula for relative atomic mass
(abundance x mass) + (abundance x mass) ... for however many isotopes there are
Mass spectrometer
An apparatus that can give information about isotopic composition of different elements and the structure of molecules.
A larger deflection occurs when?
The atom has a higher charge and a smaller mass
Detection
Ions with particular mass/charge ratio are recorded on a detector. The mass and relative amounts of ions present are measured
Line Spectrum
Discrete narrow bands of colour. Only shows discrete energy levels and specific colours/wavelengths/frequencies
Continuous Spectrum
Full spectrum of light, each colour merging into the next (no gaps). Shows all energy levels and colours/wavelengths/frequencies
Ionisation energy
The minimum energy required to remove an electron from the ground state of a gaseous atom/ion/molecule
When do lines converge in the line spectrum?
At higher energies (energy levels inside the atoms are closer together)
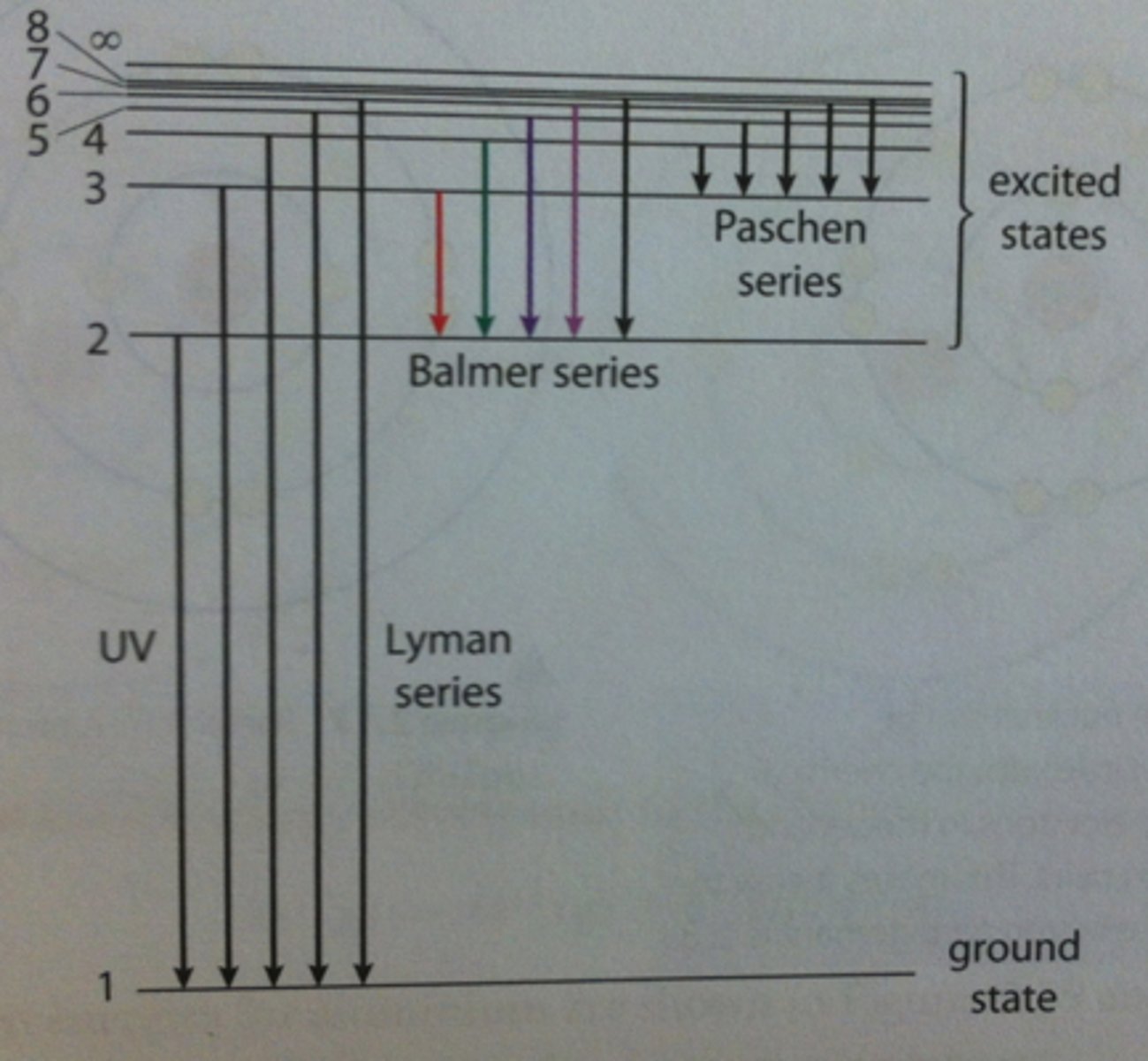
Balmer series
Visible light. Energy levels 7-2
Radioisotope
An unstable isotope of an element, which undergoes radioactive decay
Alpha particle
Emitted by nucleus when there are too many protons in it; it is not stable. Composed of two protons and two neutrons.
Beta particle
Emitted by nucleus when there are too many neutrons. Composed of electrons
Carbon-14 dating
Living creatures have particular isotopes of Carbon, which remains constant throughout life. When dead, these isotopes start to decay into nitrogen. This decay (half-life) can be measured and thus the dead creature can be dated back to when it lived.
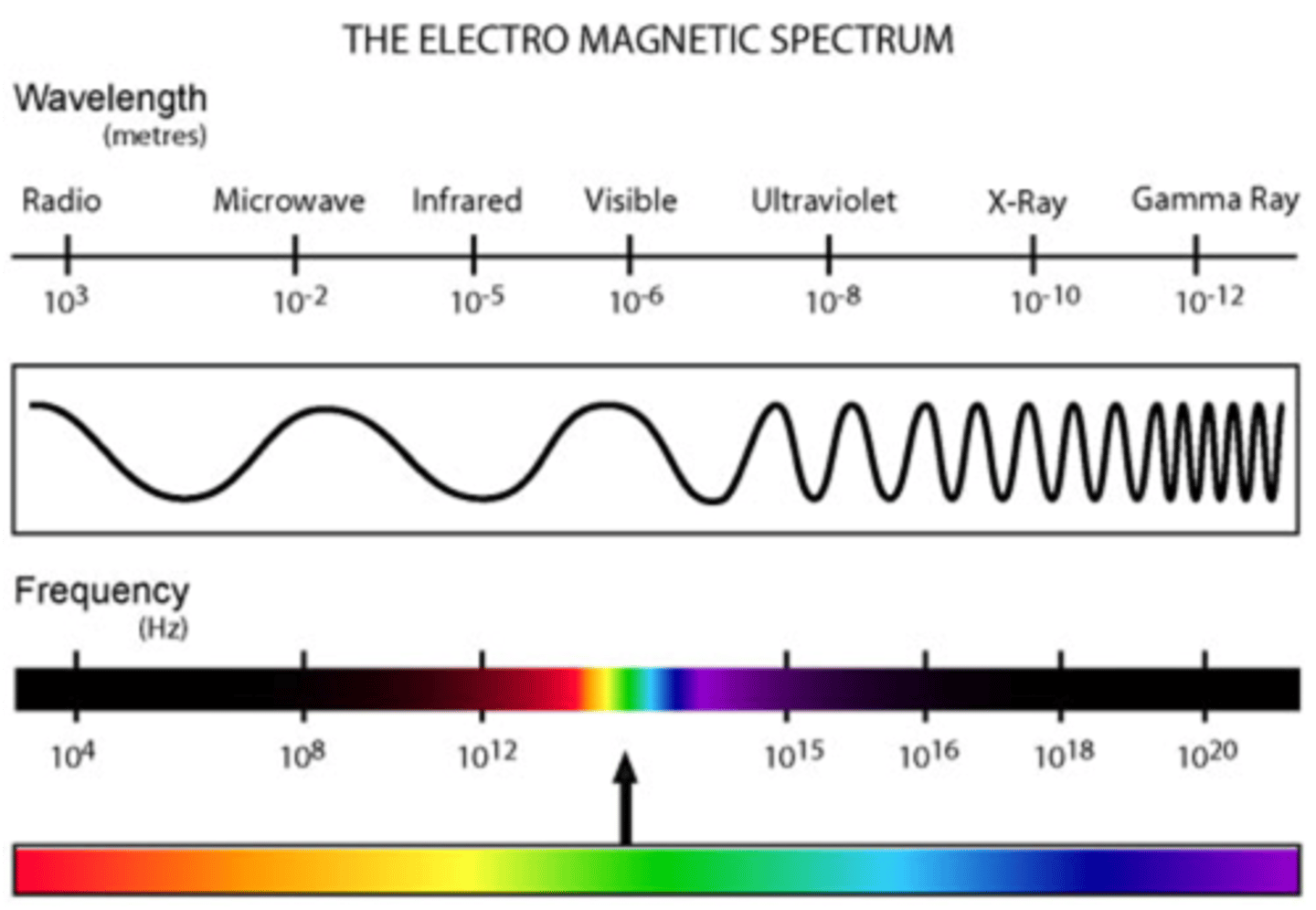
Electromagnetic spectrum
arrangement of electromagnetic radiation. including radio waves, visible light , gamma rays, X rays, ultraviolet waves, infrared waves, and microwaves--according to their wavelengths
Excited state
The unstable energy of an electron, which is disposed of when the electron falls down to the ground state
Ground state
The stable energy of an electron
Valence electrons
Outer shell electrons
Ion
When electron number differ from proton number, the atom carries a charge.
Cation
Positive Ion
Anion
Negative Ion
Mass spectrum
A graph with % abundance plotted against mass/charge, gained as a result from the mass spectrometer.
Electron arrangement
Organization of electrons in an atom by increasing energy shells.
Frequency
the number of complete wavelengths that pass a point in a given time
Wavelength
the length from one crest to another
First ionization energy
The minimum energy required to remove one mole of electrons from one mole of gaseous atoms in their ground state.
Electron configuration
the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms
sub-level
s, p, d, f; denotes shape of orbital
How many electrons can there be occupied in a s orbital?
2
State the maximum amount of electrons that can fill up a p orbital.
6
State the maximum amount of electrons that can up a d orbital.
10
State the maximum amount of electrons that can up a f orbital.
14
What is the Heisenberg uncertainty principle?
State the principle that states the following: it is impossible to determine accurately both momentum and position of a particle simultaneously. The more we know about the position, the less we know about the momentum, and vice versa. We can calculate the PROBABILITY of finding an electron in a given region of space within the atom.
Atomic orbitals
The regions around the nucleus within which the electrons have the highest probability of being found
What is the Pauli exclusion principle?
Paired electron will have opposite spin, as this reduces the mutual repulsion between the paired electrons.
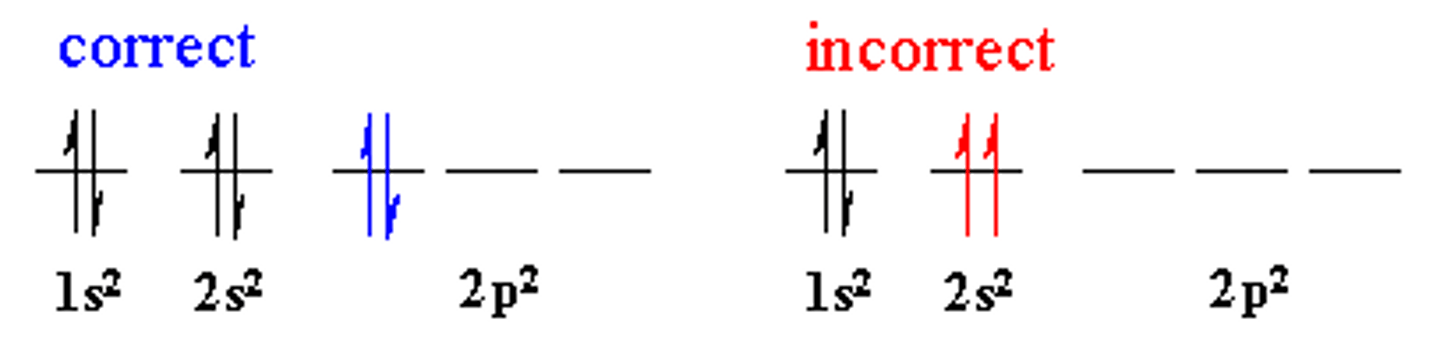
What does the Aufbau principle state?
The principle that states that electrons fill the lowest orbitals first.

What does Hund's rule state?
electrons fill degenerate orbitals as to give the maximum number of electrons with parallel spins.
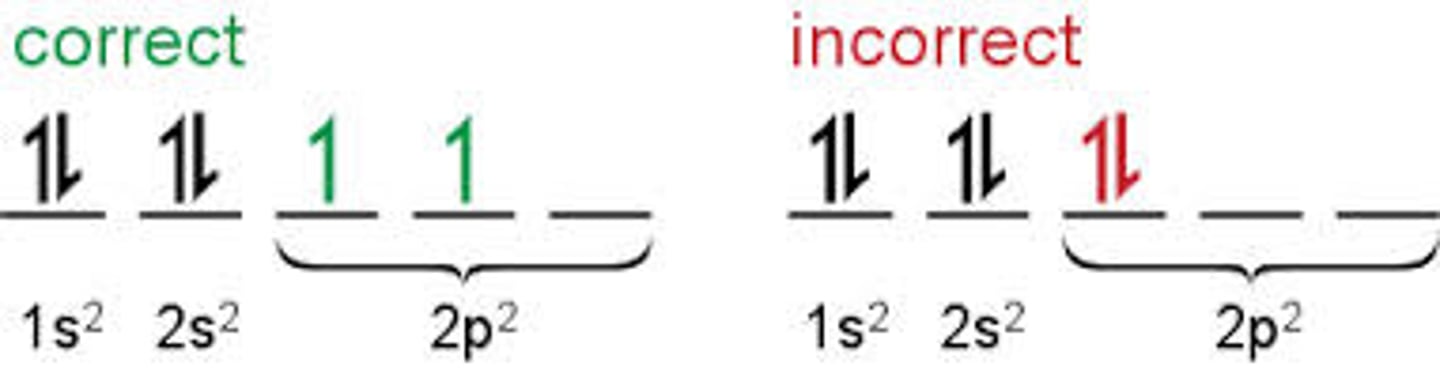
Outline the prperty of electrons that is Electron spin?
An intrinsic property of electrons that causes each electron to behave as if it were spinning about an axis. The spinning charge generates a magnetic field whose direction depends on the direction of spin.
Proton: type of particle, charge, location & mass
Sub-atomic particle, charge of 1+, located in the nucleus, weighs approximately 1amu
Neutron: type of particle, charge, location & mass
Sub-atomic particle, charge of 0, located in the nucleus, weighs approximately 1amu
Electron: type of particle, charge, location & mass
Sub-atomic particle, charge of 1-, located outside the nucleus in different energy levels, weighs 0amu or 1/1836
How the mass spectrometer works
Particles are vaporized, released into machine, are bombarded by electrons until they become positively charged, their breakdown is accelerated by an electrical field, certain masses land on the deflector, based on what goes through and what doesn't, the machine categorizes the particles
When electrons absorb energy they
Go to a higher energy level, when they return they release a photon.
S-block
Groups 1-2
D-block
Groups 3-12
P-block
Groups 13-18
Orbital
A region of space in which an electron may be found
Orbital diagram
A diagrammatic representation of electron configuration showing electrons as arrows in boxes

Quantum theory
Energy can be absorbed or emitted in 'packets' or quanta
Stability
The amount of energy possessed by a particle. The greater the stability, the lower the energy of that particle and the lower the chance of it reacting to form another species
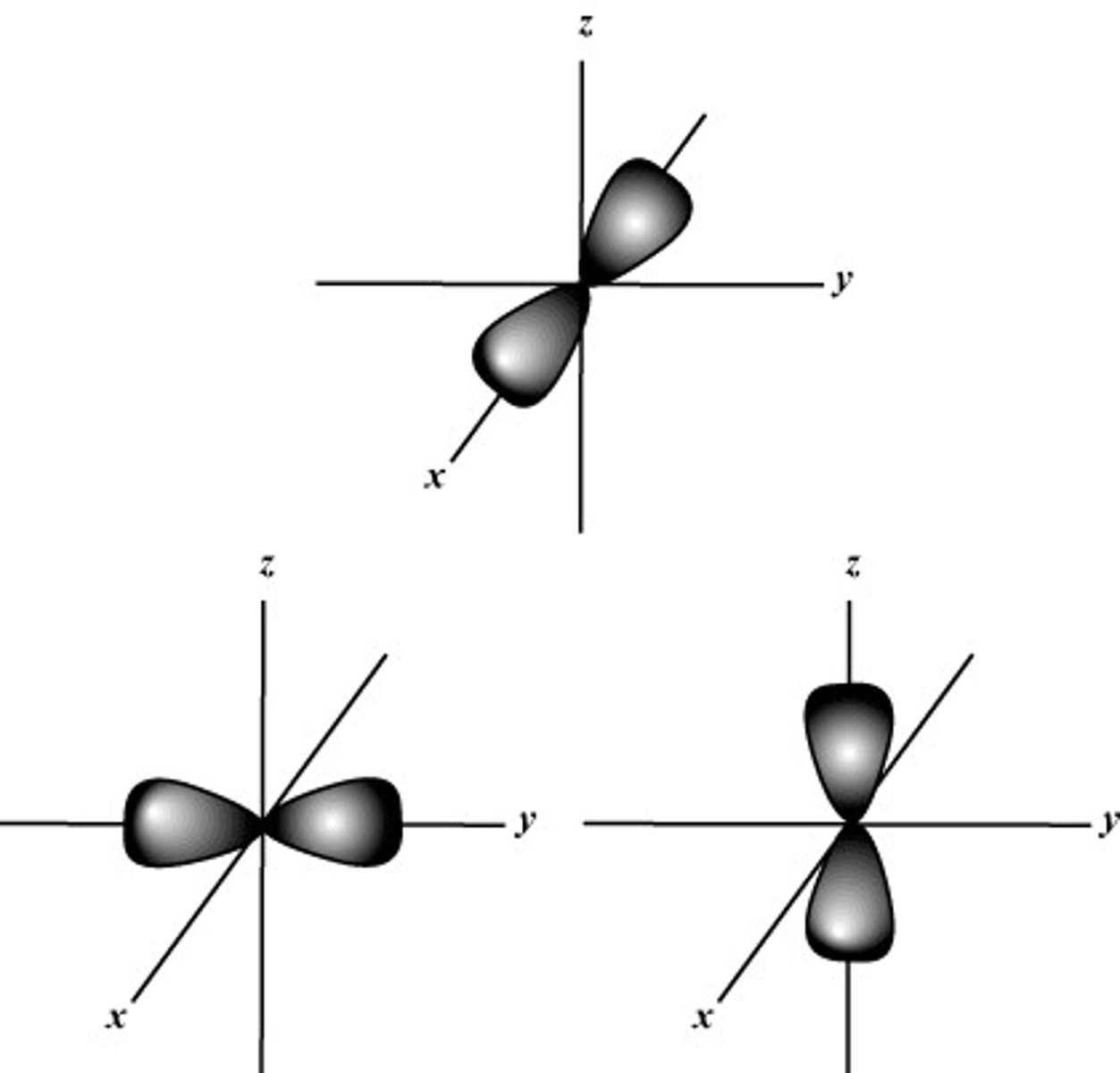
S Orbital shape
P Orbital shape
What's a photon?
a tiny particle or packet of light energy
Schrödinger's equation
mathematical equation that describes electron shells more as diffused clouds of probability that relate to a wave function than a sharply defined orbital.
What are two exceptions to the Aufbau principle?
The electronic structures of chromium and copper do not follow the pattern - they are anomalies.
Chromium - 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1
Copper - 1s2, 2s2, 2p6, 3s2. 3p6, 3d10, 4s1
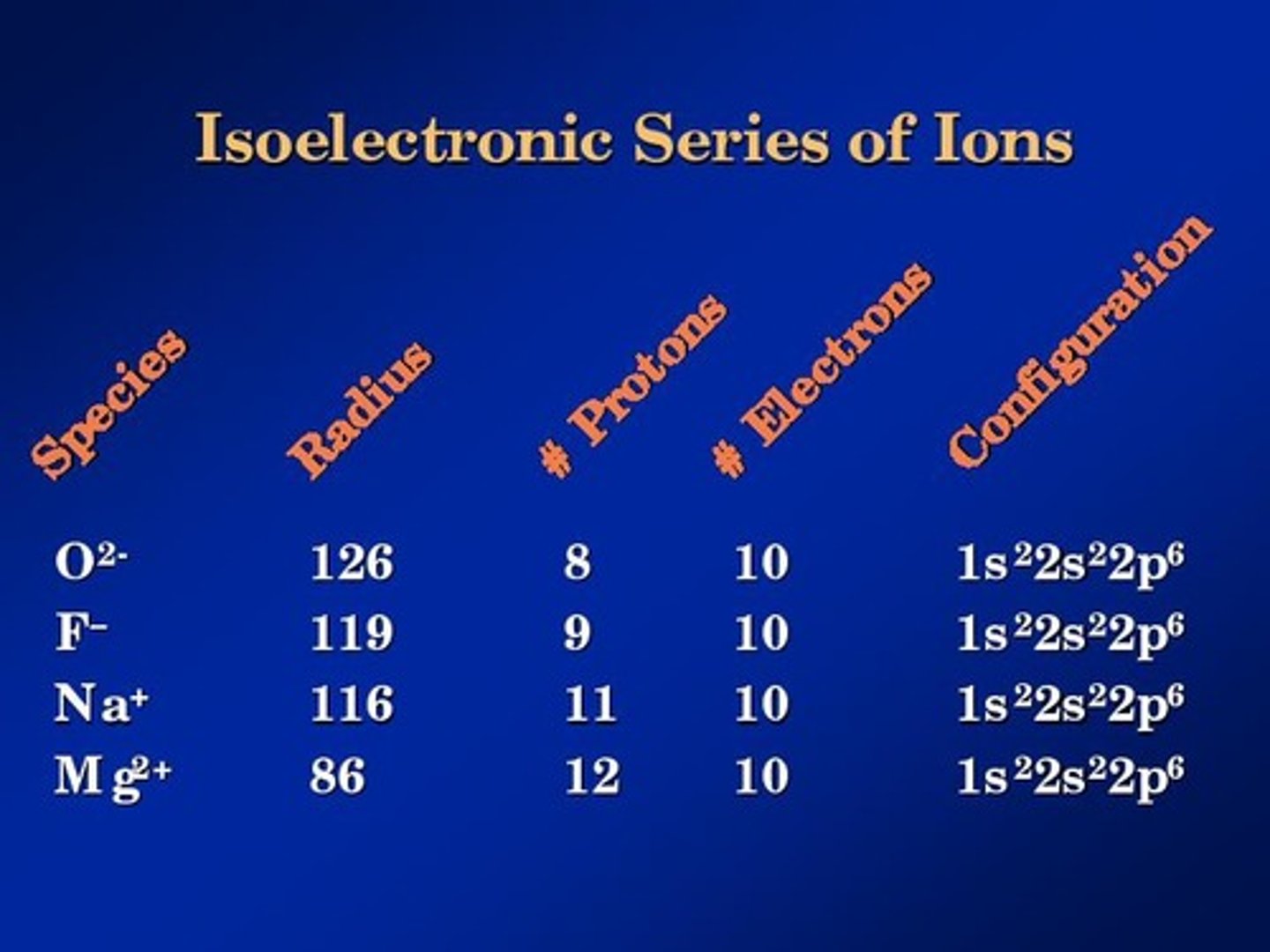
Isoelectronic Structure
Ions that contain the same number of electrons, therefore have the same electron configuration
de Broglie's equation
An equation that describes the wavelength of a moving particle; it predicts that all matter exhibits wavelike motions. Shows that macroscopic particles have too short a wavelength for their wave properties to be observed.
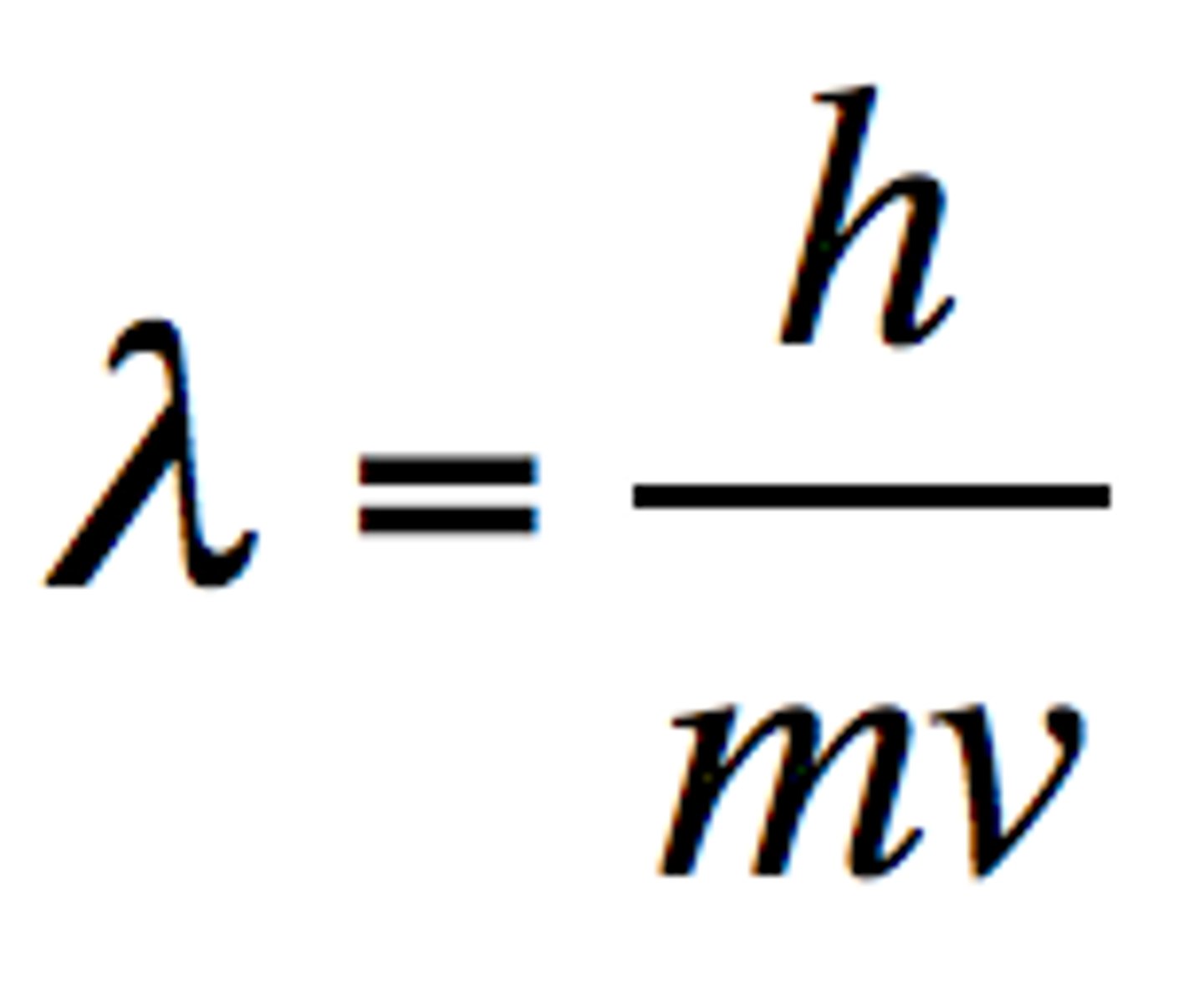
The value of the first ionization energy depends upon two main factors:
1. The size of the nuclear charge
2. The energy of the electron that has been removed (this depends upon its distance from the nucleus)
Limit of Convergence
Lines converge at the higher energies in the emission spectrum for hydrogen creating a continuum.
Past this continuum electrons are removed from the atom, which is ionization. Ionization of an atom occurs when an electron gains energy to move from n=1 to n=∞
Why is there a general increase in ionisation energies across a period?
The positive nuclear charge increases from left to right. The electrons are added to the same shell and electrons in the same shell do not shield each other very vell from the positive charge of the nucleus. Therefore the outer electrostatic force on the electrons increases
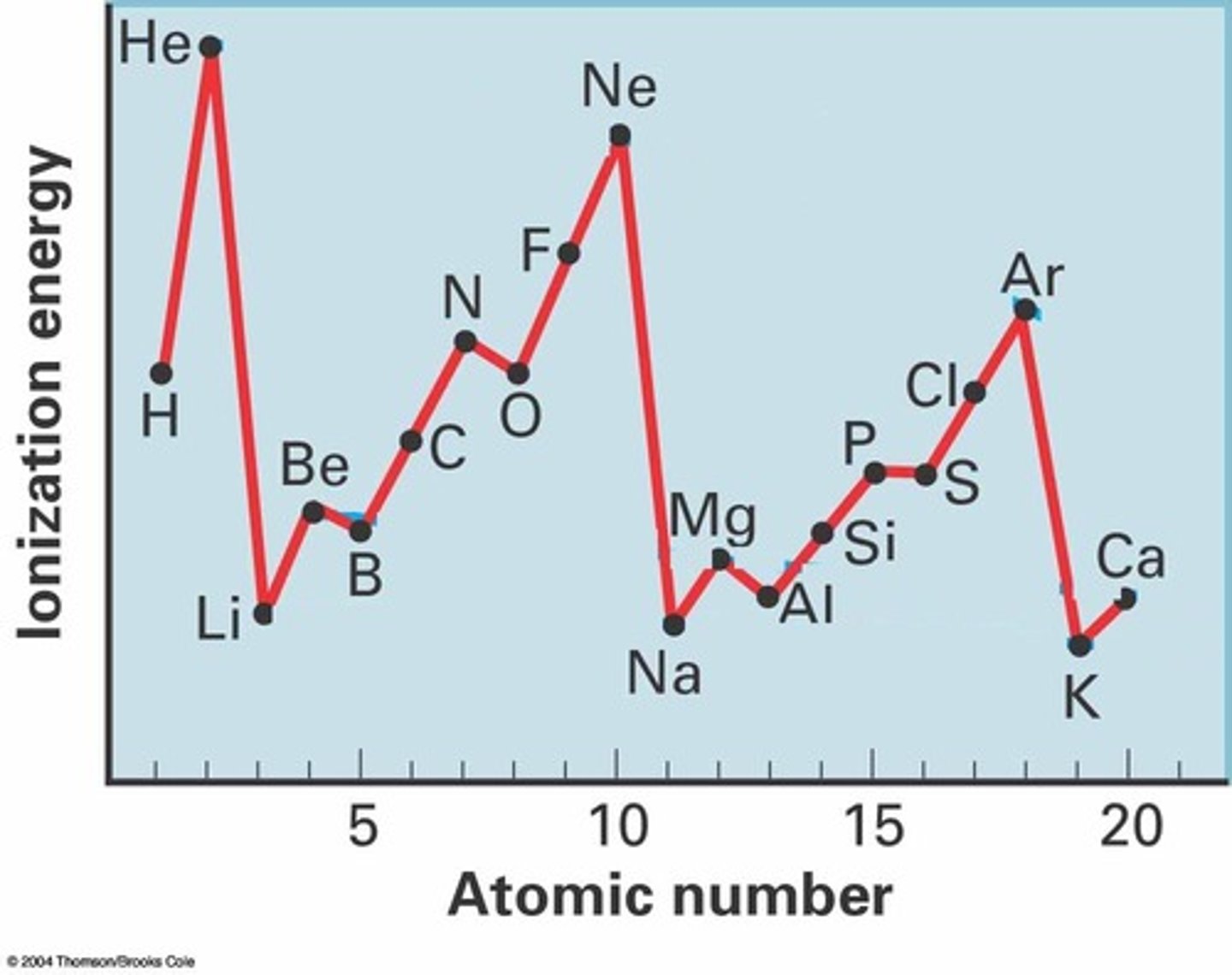
Exception to Period Trends
The first ionisation of Al is less than that of Mg, despite the increase in the nuclear charge. The reason for this is that the outer electron removed from Al is in a higher sub-level: the electron removed from Al is a 3p electron, whereas that removed from Mg is a 3s. If the 3p electron removed from S is a paired electron, whereas the 3p electron removed from P is an unpaired electron. When the electrons are paired the extra mutual repulsion results in less energy being required to remove an electron, hence a reduction in the ionisation energy.
Successive Ionization energy
ionization energy increases as you take away electrons
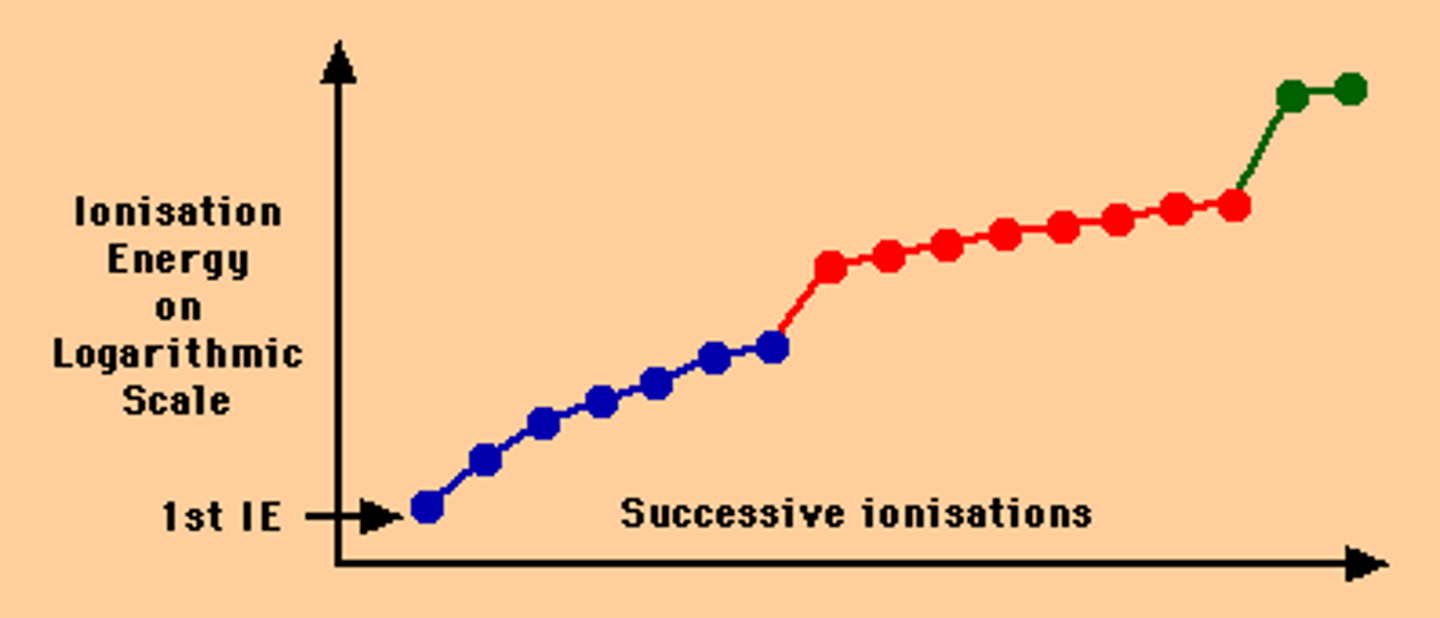
Trends down a Group
Ionization energy decreases going down a Group.
Energy Level Diagram for Hydrogen
% of heavier Isotop
(Ar- Mass of lighter I)/(Difference in Mass No)
How do we observe the electromagnetic spectrum?
A discharge tube allows our eyes to differentiate the colours and their separate wavelengths.
How do the series of lines occur?
When the electrons fall to different levels
Why does Boron have a lower first ionisation energy than Beryllium?
Boron has an electron in the 2p sublevel. Wheras the outer electron is in a 2s orbital in Be. The 2p sublevel is higher in energy than the 2s orbital and therefore less energy is required to remove the electron in the higher energy level.