short answers
1/75
Earn XP
Description and Tags
biology saq's
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
name the key anatommical parts of the nephron involved in filtration, reabsorption, and secretion and name the events that occur within each region
glomerulus — filters small solutes from the blood
PCT — reabsorbs ions, water, and nutrients; removes toxins and adjusts filtrate pH
descending loop of Henle — aquaporins allow water to pass from the filtrate into the interstitial fluid
ascending loop of Henle — reabsorbs Na+ and Cl- from the filtrate into the interstitial fluid
DCT — selectively secretes and absorbs different ions to maintain blood pH and electrolyte balance
collecting duct — reabsorbs solutes and water from the filtrate
describe the process of filtration, reabsorption, and secretion in the kidney
glomerular filtration: water and solutes smaller than proteins are forced at high pressure through the glomerular capillary walls and pores of the glomerular capsule into the renal tubule.
tubular reabsorption: water, glucose, amino acids, and ions are transported out of the filtrate into the tubule cells and then into the capillaries.
tubular secretion: H+, K+, creatine and drugs are removed from the peritubular capillaries and secreted back into the filtrate within the renal tubular lumen.
describe the CCM
due to glomerular filtration, ions, water, and nutrients enter the nephron
they are reabsorbed in the PCT
this creates higher osmolarity within the ECF and so more water leaves the descending limb via osmosis
this increases the concentration of ions in the descending limb as it goes further into the medulla
ions are pumped out of the ascending limb to ensure more water is reabsorbed in the descending limb
this ensures concentrated urine is formed
describe the function of ADH after dehydration
water loss is detected by the hypothalamus due to increased plasma osmolarity
the posterior pituitary releases ADH into the bloodstream
ADH binds to the V2 receptor on the basolateral membrane on the DCT which increases the number of aquaporins that are inserted into the membrane (signal transduction)
this increases the amount of water that is reabsorbed into the bloodstream, thereby diluting blood
plasma osmolarity is returned to its normal level
this is detected by the hypothalamus and as a result ADH secretion is reduced
describe the effect of temperature on enzyme activity
at low temperature, enzyme activity is low.
molecules have low speeds with reduction in frequency of collision, molecules do not have sufficient energy to overcome activation energy
between 0-40 degrees enzyme activity increases with increasing temperature
molecules move faster and collide more frequently and successfully
enzyme activity peaks at optimum body temperature
above 40 degrees, most enzymes denature as intra/intermolecular bonds are broken and tertiary structure is damaged no longer forming ESC
describe the 3 meningeal layers and their function
the meninges cover the brain and spinal cord & protect the neural tissue from bruising against the bones of the skeleton
dura matter — outermost layer and adheres the skull, thickest layer, and is associated with veins that drain blood to the venous sinuses
arachnoid layer — loosely connected to the inner membrane (forms subarachnoid space)
pia matter — innermost layer, adheres to brain surface, many tiny blood vessels
compare and contrast the main features of skeletal and cardiac muscle tissue
skeletal — attach and help move bone, voluntary, composed of muscle fibre with a striated appearance, multi-nucleated
cardiac — shorter, thicker, branched, involuntary, striated, uni-nucleated, found in the heart only
describe the function of the troponin complex
tropomyosin — prevents myosin and actin from binding at rest
troponin I — blocks myosin-binding sites
troponin C — binds to calcium to initiate muscle contraction
troponin T — facilitates the binding of tropomyosin to troponin
describe what is excitation-contraction coupling
refers to the mechanism that converts action potentials to muscle fibre contraction
ACh releases binds to nicotinic receptor found on motor endplate
Na+ released into T-tubule depolarising it and initiating an action potential
DHP receptor at end of T-tubule undergoes conformational change
RyRs receptor (mechanically attached to DHPR) on sarcoplasmic reticulum opens up and releases Ca2+
increased intracellular Ca2+ attaches to troponin C and allows cross-bridge cycle to initiate
what constitutes the upper and lower respiratory tracts?
nose, nasal cavity, pharynx, larynx, trachea, bronchi, terminal bronchioles, respiratory bronchioles, alveolar ducts, alveoli
explain the stages of inspiration during pulmonary ventilation
the diaphragm and external intercoastals contract, diaphragm descends and rib cage rises, thoracic cavity volume increases decreasing its pressure, alveolar volume increases (decreasing pressure) and alveolar pressure becomes less than atmospheric pressure, a pressure gradient is formed between the atmosphere and lungs, air flows from the atmosphere into the alveoli and gas exchange occurs
describe the second line of defence
innate/non-specific
phagocytes / macrophages
natural killer cells
inflammation
complement system
interferons
fever
describe the difference between active and passive immunity
active — our own immune system is responsible for protecting us against a pathogen and producing antibodies (uses APC, T-helper, T-killer, T-memory, T-suppressor cells & memory B cells and plasma cells)
passive — antibodies artificially produced outside the body and injected into the body (no contact with antigen)
describe the general structure of a virion?
viral genome
nucleic acid core
capsid/protein coat
nucleocapsid
glycoprotein envelope
surface protein
describe the general structure of prokaryotes
DNA in nucleoid
peptidoglycan cell wall
plasma membrane
70s ribosomes
cytoplasm
glycocalyx gel-coating
appendages (flagellum, fimbriae, conjugation pilus)
define bacterial conjugation and describe this process
conjugation is the process where one bacterium transfers genetic material to another through direct contact
a pilus forms between 2 bacteria
donor passes DNA to recipient through pilus
requires F plasmid (fertility factor)
F+ has F plasmid and donates to F- cells
what is an energy profile and how do energy profiles of exothermic reactions differ from endothermic ones?
energy profiles show the energy of reactants and products
exothermic — products have less energy than reactants, releases energy to surroundings
endothermic — products have more energy than reactants, absorbs energy from surroundings
transition state point on graph with highest energy
activation energy is energy barrier between converting substrate to product
reversibe competitive vs. non-competitive inhibition
competitive — bind to same site as substrate, can be displaced by increasing substrate concentration, do not effect Vmax but Km increases
non-competitive — bind to allosteric site, not displaced, Vmax reduced, Km stays the same
what are two models that explain how enzymes and substrates interact and how do they differ?
lock & key model — proposes that the enzyme and substrate fit together like a lock and key and hence only the substrate fits the active site forming an ESC
induced fit model — suggests that the active site detects the substrate and molds around it and therefore make it more accurate
what are cofactors and coenzymes? give an example of each
cofactor — non-protein inorganic substance that binds to enzymes to enhance their activity, e.g. Fe2+, magneisum, zinc, cobalt
coenzyme — an organic substrate that is necessary for the functioning of an enzyme, e.g. vitamin derivatives such as CoA, NAD, FAD
what are the factors that affect enzyme activity and which of these factors can cause denaturation?
the concentration of the enzyme, the concentration of the substrate, temperature, pH, presence of an inhibitor
temperature & pH can cause denaturation by breaking the weak intermolecular bonds within the 3D tertiary structure
what are the key functions of saliva?
protects the oral cavity by coating it with a proline-rich protein (pellicle)
to ease swallowing
to moisten and lubricate the mouth
to dissolve food molecules so they react with taste receptors
chemical digestion of polysaccharides with amylase
what are the main functions of the stomach?
mechanical and chemical digestion
temporary storage of food
secrete intrinsic factor to absorb vitamin B12
regulate the release of chyme into the duodenum
describe the cells of the stomach and what they secrete
parietal cells — gastric acid (HCl)
chief cells — pepsinogen (pepsin precursor)
goblet cells — mucus
D cells — somatostatin (inhibits gastric acid release)
G cells — gastrin (induces gastric acid release)
direct vs. indirect pathway
acid secretion is stimulated by acetylcholine (neural), histamine (paracrine), and gastrin (endocrine)
direct pathway — ACh binds directly to M3 receptor, histamine binds to H2 receptor, gastrin binds to CKK2 receptor on parietal cells
indirect pathway — ACh and gastrin bind to ECL cell and stimulate histamine release that then binds to parietal cells
both pathways end up in the secretion of H+ into the lumen via a K+ co-transporter
how is the structure of the small intestine adapted for its function?
rich blood supply
lacteals
intestinal crypts
single-layer epithelial cells
microvili
several membrane proteins
chyme forced into a circular motion aiding digestion and absorption
describe the differences between DNA and RNA
DNA — double stranded, thymine base, found in the nucleus and mitochondria, more stable (long-term storage), deoxyribose sugar, converted to mRNA during transcription
RNA — single stranded, uracil base, found in the cytoplasm, nucleus, and ribosomes, less stable ribose sugar, translated into a protein
what is a codon and why are they considered degenerate?
a codon is a series of triplet nucleotides that corresponds to an amino acid
there are 64 different codons (61 of which code for amino acids) — 1 stop AUG (methionine), 3 stop UAA UAG UGA
the genetic code is considered degenerate as more than one codon can code for a specific amino acid
describe the structure of transfer RNA involved in eukaryotic gene expression
tRNA has distinct three hairpin loops (T loop, D loop, and anti-codon loop) that form a three-leafed clover
anti-codon loop recognises mRNA codon
aminoacyl-tRNA is covalently bonded to an amino acid
describe the difference in gene expression in eukaryotes and prokaryotes
eukaryotes — controlled at multiple levels: epigenetics, transciption, post-transcriptional modification, translation, and post-translation, 60s and 40s subunits, occurs in the nucleus (transcription) and cytoplasm (translation)
prokaryotes — controlled at transcription, 50s and 30s subunits, transcription and translation occurs in the cytoplasm
what are post-transcriptional modifications, and why do they occur?
before modifications, mRNA is called pre-mRNA
a 5’ cap is added to the beginning of the chain to prevent degradation
a 3’ poly-A tail is added to the end to prevent degradation and to help move the mRNA to the ribosomes
introns (non-coding bits) are spliced and removed while exons (coding bits) are ‘glued’ together using ligases — this is known as gene splicing
mRNA is now considered mature
what nutrients are absorbed in the small intestine?
glucose, amino acids, lipids, electrolytes, water, vitamins, minerals
how are carbohydrates digested and absorbed?
first digested by amylases in saliva
enzymes released by brush border (lactase, sucrase, maltase) breaks down disaccharides to monosaccharides — glucose, galactose, fructose
monosaccharides transported into bloodstream by means of a cotransporter with Na+ (SGLT) set up by Na+/K+ ATPase
monosaccharides then leave the basolateral membrane by simple/facilitated diffusion down its concentration gradient
where and how are proteins broken down and absorbed?
broken down into polypeptides in the stomach by pepsin
these polypeptides are further broken down into oligopeptides in the small intestine by proteases secreted by the pancreas (trypsin & chymotrypsin)
oligopeptides broken down into amino acids by pancreatic enzyme carboxypeptidase and converted into free amino acids by aminopeptidase
these free a.a. are transported into epithelial cells by secondary active transport coupled with the movement of Na+
these a.a. are then moved into the bloodstream by transport proteins and ATP
where and how are fats digested and absorbed?
occurs mainly in the small intestine
pancreatic enzyme lipase which breaks down fats into monoglycerides and free fatty acids
emulsification of larger fat droplets into smaller ones by bile increases surface area for lipase to act on
small fat droplets bound to bile salts (makes micelles) then lose the bile salts and enter the cell passively
these fats binds to proteins in the golgi complex to form chylomicrons which are then removed from the cell via lacteals
what do pancreatic juices include and what is their function?
amylase — breaks down carbohydrates into monosaccharides
lipase — breaks down fats into glycerol and fatty acids
ribonuclease & deoxyribonuclease — breakdown of nucleic acids into mononucleotides
proteolytic enzymes (trypsin, chymotrypsin, carboxypeptidase, elastase) — proteins into small peptides into a.a.
+ water, bicarbonate ions (neutralise gastric acid)
what are the key functions of the liver?
metabolism
storage of vitamins and minerals
production of bile
production of alkaline fluids
breakdown of waste products
detoxification
what are the key functions of bile, where is it secreted from, and where is it stored?
made and released by the liver into the duodenum
stored in the gallbladder
provides an alkaline fluid to neutralize stomach acid
bile salts aid in digestion of fats (emulsification)
increases surface area of lipids allowing absorption of fat-soluble vitamins
what is the key function of the large intestine and how does it perform it?
to absorb water and electrolytes:
movements of the large intestine are slow and sluggish to allow for absorption
chyme that enters is isotonic so requires energy (Na+/K+ ATPase)
water enters chyme by osmosis
alveolar type i vs. ii cells
type 1 — involved in gas exchange, cover 95% of alveolar surface area, thin structure allows for shorter diffusion distances, susceptible to damage by emphysema
type 2 — cuboidal cells, secrete surfactant which prevents collapse of alveoli and reduces tension, repair alveolar epithelium, more resistant to damage
spinal nerve pairs
31 spinal nerve pairs in total:
8 (cervical) C1-C8
12 (thoracic) T1-T12
5 (lumbar) L1-L5
5 (sacral) S1-S5
1 (coccygeal) C0
lung volumes?
→ tidal volume (TV)
‘quiet breathing’ rate, average resting value of 500ml
→ inspiratory reserve volume (IRV)
amount of air that can be maximally inspired above TV, average value of 3000ml
→ inspiratory capacity (IC)
tidal volume + inspiratory volume, TV + IRV
→ expiratory reserve volume (ERV)
extra volume of air that can be expired after TV, average of 1000ml
→ residual volume (RV)
volume of air remaining in lungs to prevent deflation, average of 1200ml
→ functional residual capacity (FRC)
expiratory reserve volume + residual volume, ERV + RV
→ vital capacity (VC)
maximum volume of air that can be moved in and out in a single breath, all the values minus residual volume
→ total lung capacity (TLC)
the maximum volume of air the lungs can hold, IRV + TV + ERV + RV
how is oxygen transported in the body?
dissolved in plasma & combined with haemoglobin
oxygen binding is cooperative, binding affinity increases as more oxygen is binded
how is CO2 transported in the body?
dissolved in plasma (5%), carbaminohaemoglobin (10%), bicarbonate (85%)
what controls the rate of breathing?
medullary respiratory centre
dorsal respiratory group at the back of the medulla spontaneously fire off neurons
pons respiratory centre
pneumotaxic area sends inhibitory signals to dorsal group
sometimes we need to breathe in deeper, the medulla mainly (as well as chemoreceptors, carotid & aortic bodies) send signals to intercostal muscles to increase ventilation
describe the main functions of the skeletal system
facilitates movement
stores and releases minerals
supports the body
protects internal organs
stores and releases fats
produces red blood cells
what are the components of the muscoskeletal system?
muscles, bone, tendons (bone to muscle), ligaments (bone to bone), cartilage, joints
describe cartilage and it’s key function
cartilage is a tough flexible connective tissue that protects bones and joints
acts as a shock absorber
prevents friction between bones
synovial fluid found in the cavities of synovial joints (viscous solution)
different layers of bone
periosteum
compact bone
spongy bone
bone marrow (yellow/red)
bone is richly vascularised and has a hydroxyapatite bone (calcified matrix)
bone cells
osteoblasts — build up bone
osteoclasts — break down bone
osteocytes — trapped osteoblasts within the calcified matrix
osteogenic cells — undifferentiated bone cells (stem cells)
what are prostaglandins?
local mediators that are stimulated when the mucosa is irritated and increase the thickness of the mucosa (as well as increase bicarbonate production)
describe the structure of skeletal muscle fibres
Each fibre is a long, cylindrical cell arranged in parallel and contain multiple nuclei.
The membrane of the muscle fibre is the sarcolemma.
The cytoplasm is the sarcoplasm, that contains many mitochondria.
Composed of myofibrils that are responsible for the striated appearance.
The sarcoplasmic reticulum is a form of endoplasmic reticulum (stores Ca2+ required for muscle contraction).
Each myofibril contains contractile proteins, which are arranged into units called sarcomeres
The main unit of the thick filament is the large motor protein myosin.
Each myosin molecule contains two identical protein chains, each with one large heavy and two light chains.
The other contractile filament in myofibrils is the thin filament, mainly composed of three proteins: actin, tropomyosin, and troponin.
describe the key stages of the cross-bridge cycle
myosin bound to actin, no adenosine triphosphate (ATP); rigor state
ATP binds to myosin head resulting in a conformational change decreasing affinity to actin
ATP bound to myosin hydrolyses to ADP + Pi which remain linked to myosin
myosin binds weakly to actin, tropomyosin moves off the binding site and power stroke begins
myosin releases ADP at the end of the power stroke and the rigor state begins again
describe the organisation of the nervous system
CNS
brain
spinal cord
PNS
sensory neurons (afferent)
motor neurons (efferent)
somatic nervous system (voluntary)
autonomic nervous system (involuntary: fight or flight + rest and digest)
describe the main catergories of neurons
afferent — carry information about temperature, pressure, light, and other stimuli from sensory receptors to the CNS
efferent — both somatic motor and autonomic have enlarged endings called axon terminals and carry signals from the CNS to muscles and glands
mixed — nerves that carry signals in both directions
interneurons — neurons that lie entirely within the CNS and often have quite complex branching processes that allow them to communicate with many other neurons
name the differeny types of glial cells and their function
CNS
microglia — act as scavengers
oligodendrocytes — form myelin sheath and associated with more than 1 axon
ependymal — create a selectively permeable barrier separating fluids of the CNS
astrocytes — take up water, K+, neurotransmitters and help form the BBB
PNS
schwann — form myelin sheath around 1 axon
satellite — support cell bodies
arrangement of the lower spinal column
→ the L2 vertebral level tapers off forming the conus medullaris
→ at the end of the spinal cord the subarachnoid space expands forming the lumbar cistern
→ this space is accessed during a standard lumbar puncture procedure
→ the filum terminale is a fibre that extends from the conus medullaris to the coccyx and it helps fixate the spinal cord
→ the spinal nerves that arise from the end of the spinal cord are known as the cauda equina
what is the blood-brain barrier
⇒ high selectively permeable brain capillaries protects brain from harmful substances
⇒ endothelial cells form tight junctions that prevent solute movement
⇒ these tight junctions form a functional blood brain barrier
describe how the brain and spinal cord are protected by bone
→ The brain is encased in a bony skull, or cranium, and the spinal cord runs through a canal in the vertebral column
→ The bony vertebrae are stacked on top of one another and separated by disks of connective tissue
→ Nerves of the PNS enter and leave the spinal cord by passing through notches between the stacked vertebrae
explain how neurotransmitters are released and removed from axon terminals
depolarisation at axon terminal opens voltage-gated Ca²⁺ channels
Ca²⁺ enters cell due to electrochemical gradient and triggers exocytosis
neurotransmitters diffuses across synaptic cleft and binds to receptor on postsynaptic cell
neurotransmitter binding initiates a response in postsynaptic cell
REMOVED:
diffuse away from the synapse separating from receptors
inactivated by enzymes, e.g. ACh broken down into acetyl CoA and choline by the enzyme acetylcholinesterase and choline can be reused at the presynaptic axon terminal
transport into adjacent glia or neurons and can be recycled to refill empty vesicles
describe the different stages of neural action potentials
action potential initiates when Na⁺ channels open and enter the neuron
this causes depolarisation and opens more adjacent Na⁺ channels
continuous entry of Na⁺ opens along the axon so that the signal does not diminish
K⁺ channels open and K⁺ leaves cell; repolarisation
additional K⁺ leaves hyperpolarising the cell
voltage-gated K⁺ channels close and cell returns to resting membrane potential
name all the functions of an astrocyte
glial cell in the CNS
highly branched
provide neurons with metabolic substances for ATP production
maintain homeostasis by taking up K+ and water
forms the BBB
source of neural stem cells
secrete neurotrophic factors
what are conditions that arise from nondisjunction?
down syndrome (trisomy 21)
edwards syndrome (trisomy 18)
turners syndrome (monosomy X)
klinefelter syndrome (XXY)
patau’s syndrome (trisomy 13)
what factors can cause nondisjunction?
failure of homologues to separate in anaphase I
failure of sister chromatids to separate in anaphase II
the resulting gamete will either possess either an extra or missing copy
the resulting offspring will have an incorrect chromosome number in every cell of the body
what is a zymogen? give an example
an inactive substance which is converted into an enzyme when activated by another enzyme, e.g. pepsinogen
anterior pituitary hormones
FSH & LH
ACTH
GH
endorphins
TSH
prolactin
what are the 3 laws of inheritence?
law of dominance
in a heterozygote, one trait will conceal the presence of another trait for the same characteristic; the dominant allele will be expressed in the phenotype
independent assortment
the alleles of two or more different genes get sorted into gametes independently of another; the allele a gamete receives for one gene doesn’t influence the allele received for another
law of segregation
during gamete formation, each gene separates from each other so that each gamete only carries only one allele for each gene
explain the process of deamination
Intake of proteins produces excess amino acid in the gastrointestinal tract, which needs to be excreted.
In the liver, the amino acid is converted to ammonia soluble in water to form alkaline fluid, which is toxic through deamination.
This toxic substance is converted to urea for safe excretion.
The protease enzyme breaks down the digested protein into amino acids in the small intestine and stomach.
After the conversion of excessive amino acid into urea, it is transported to the kidney via the bloodstream, where the blood is filtered, and urea is excreted through urine
explain how vitamin B12 is absorbed
B12 comes from animal proteins
HCl in the stomach releases B12 from proteins
free B12 binds to R-proteins (glycoproteins) secreted by the salivary glands and stomach
pancreatic enzymes in the duodenum break apart B12 and R-protein
free B12 binds to intrinsic factor
B12-IF is absorbed in the ileum by receptor-mediated endocytosis
describe the lytic & lysogenic cycles
lytic:
capsid attaches to receptor on the cell membrane
viral DNA penetrates the host cell
viral components are synthesised
new viruses are assembled
new viruses leave the host cell
lysogenic:
capsid attaches to receptor on the cell membrane
viral DNA penetrates the host cell
viral DNA is integrated into bacterial DNA and is passes on when bacteria reproduce
daughter cells now carry viral DNA
describe the viral life cycle
attachment / absorption
entry into cell
release of viral genome
replication of genetic material
viral assembly
viral release
budding
cell lysis
exocytosis
what are the 3 types of horizontal gene transfer? briefly explain them
bacterial conjugation — F+ to F- (fertility factor) through a pilus
transduction — phage infection
transformation — bacteria takes in DNA fragments of a cell that died
what is the effect of viruses on cells?
cell death (cytopathic effect)
viral latency (virus remains in cell but dormant)
viral transformation (cell properties are changed so its malignant)
types of immunoglobins
IgM — largest pathogen (destroys bacteria/contains disulfide bonds)
IgG — coats pathogens (speeds uptake)
IgE — protects against parasitic infections
IgD — attaches to B cells initiating early B cell response
IgA — found in tears & saliva
how are antibodies made?
antigen recognition by macrophage → antigen presentation → B cells undergo clonal selection → produce plasma cells which differentiate → antibodies produced by plasma cells
CD4+ T cells trigger an immune response by binding to the antigen-presenting cell
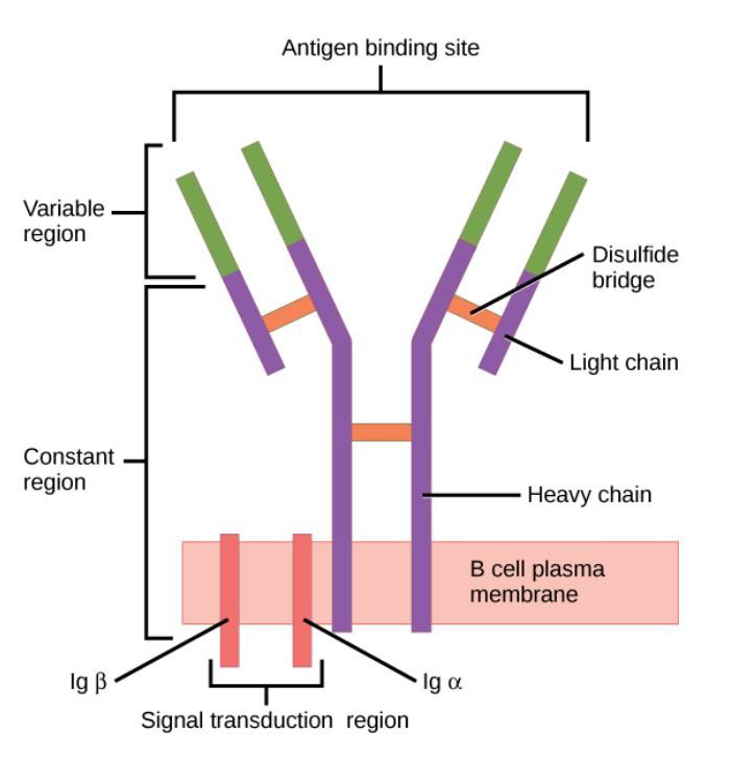
describe the structure of an antibody