week 8(/9)(1) endocrine: type 1 diabetes, pancreas histology
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
what are the 3 hormone groups, what are they derived from, and what are examples of each?
Amine
–Derived from a single amino acid
–Adrenaline/noradrenaline/dopamine (tyrosine)
Peptide
–Derived from multiple (3-200) amino acids
–All pituitary hormones
–Insulin, leptin, ghrelin
Steroid
–Derived from cholesterol
–Glucocorticoids/mineralocorticoids/androgens/progesterone
how do steroid hormones act?
they bind to intracellular receptors
receptor/ligand complex binds DNA to affect transcription
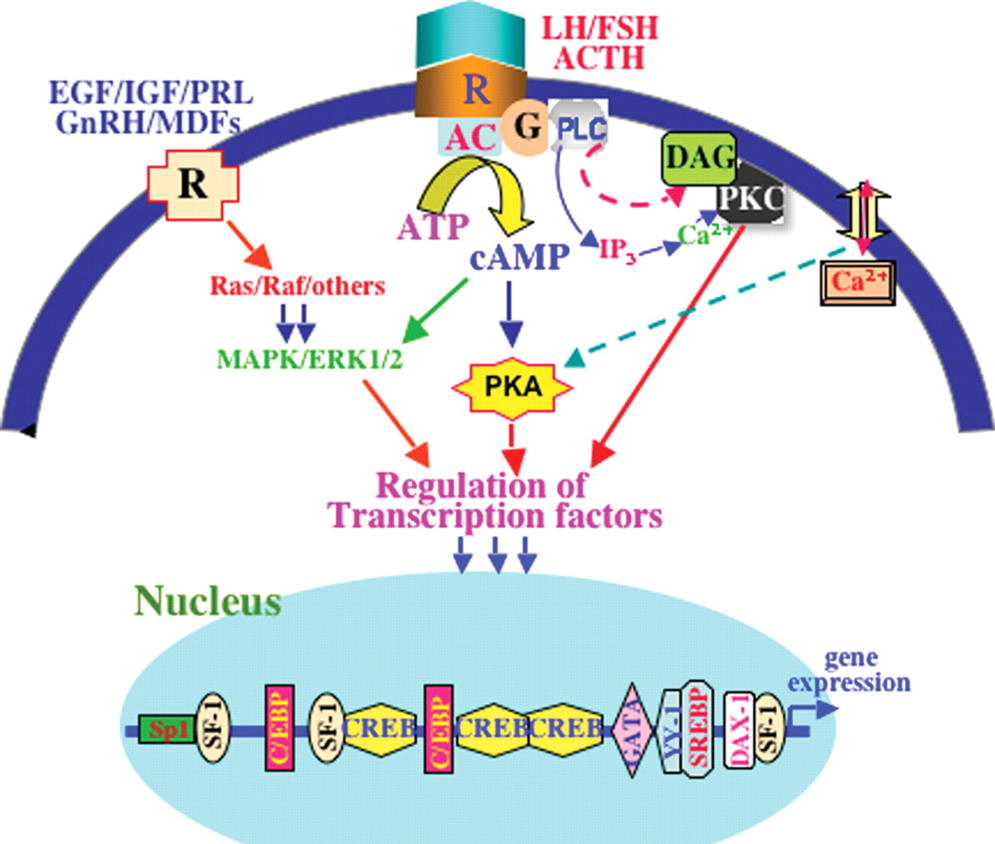
how do G protein coupled receptors act?
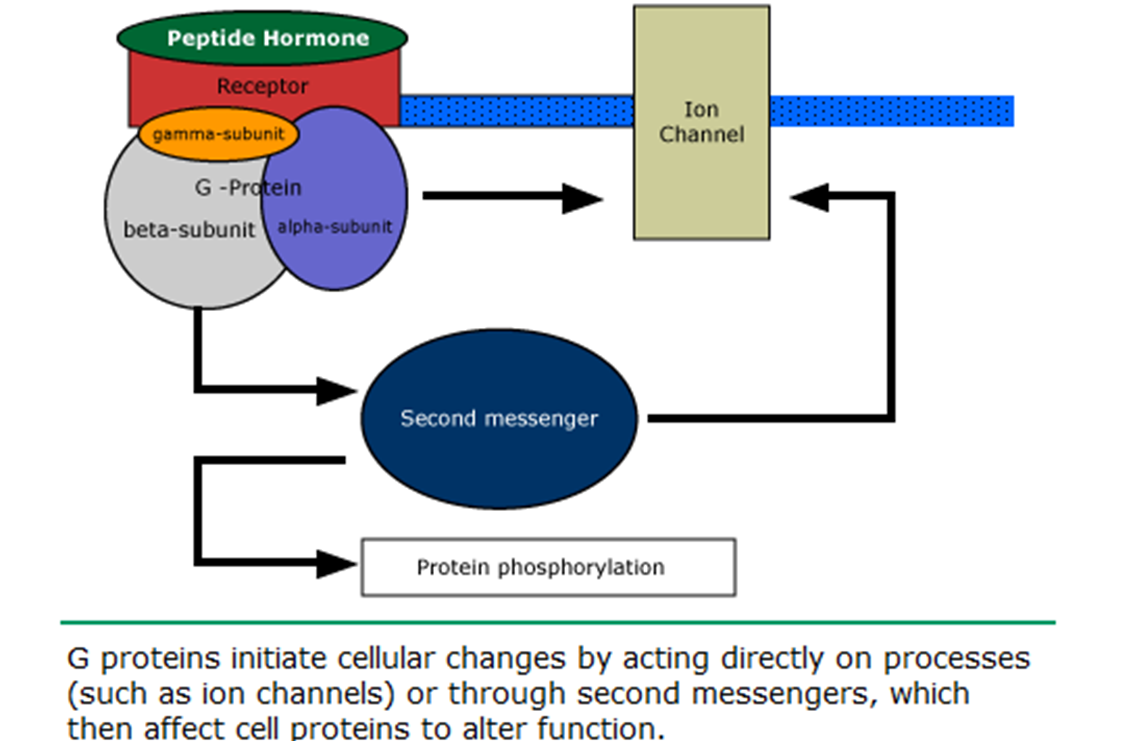
how do peptide hormone act?
peptide hormone can bind with G-protein coupled AND tyrosine kinase receptors, …
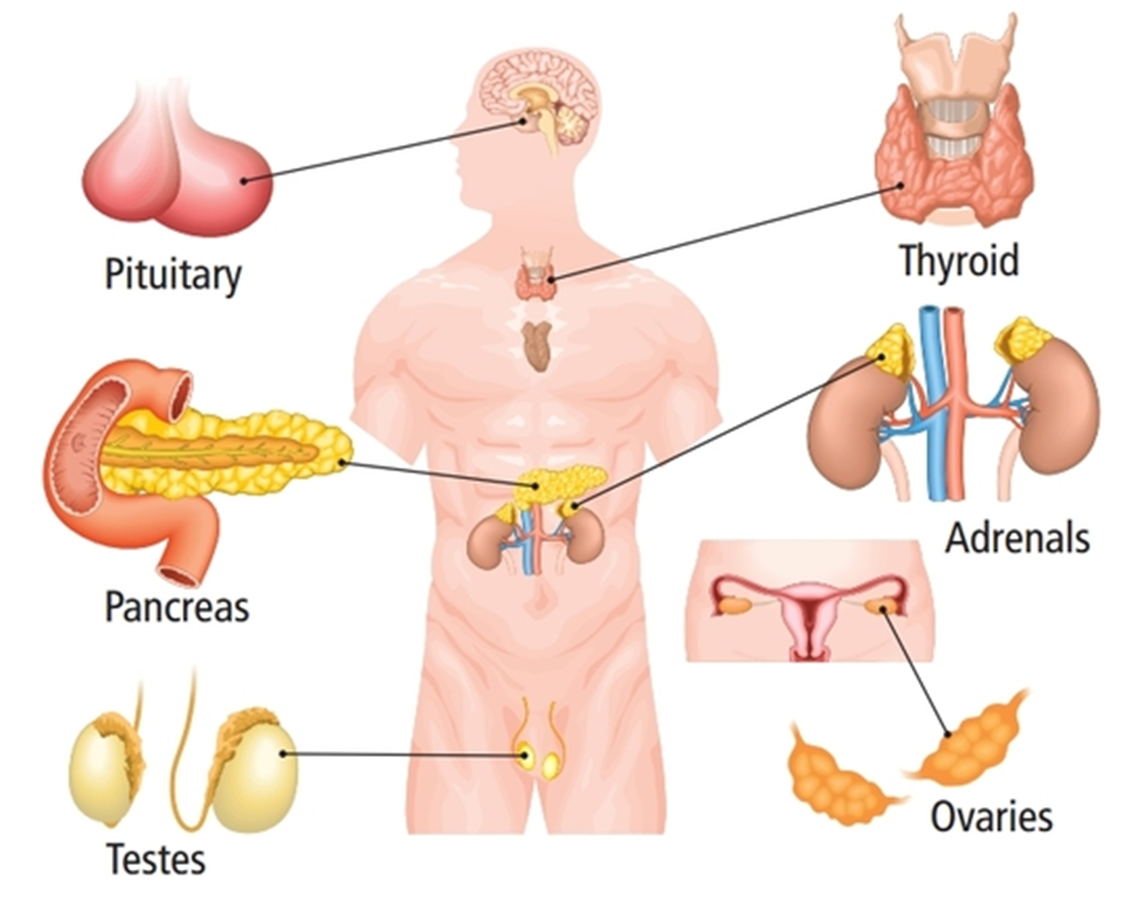
name the endocrine glands
thyroid, adrenals, ovaries, testes, pancreas, pituitary
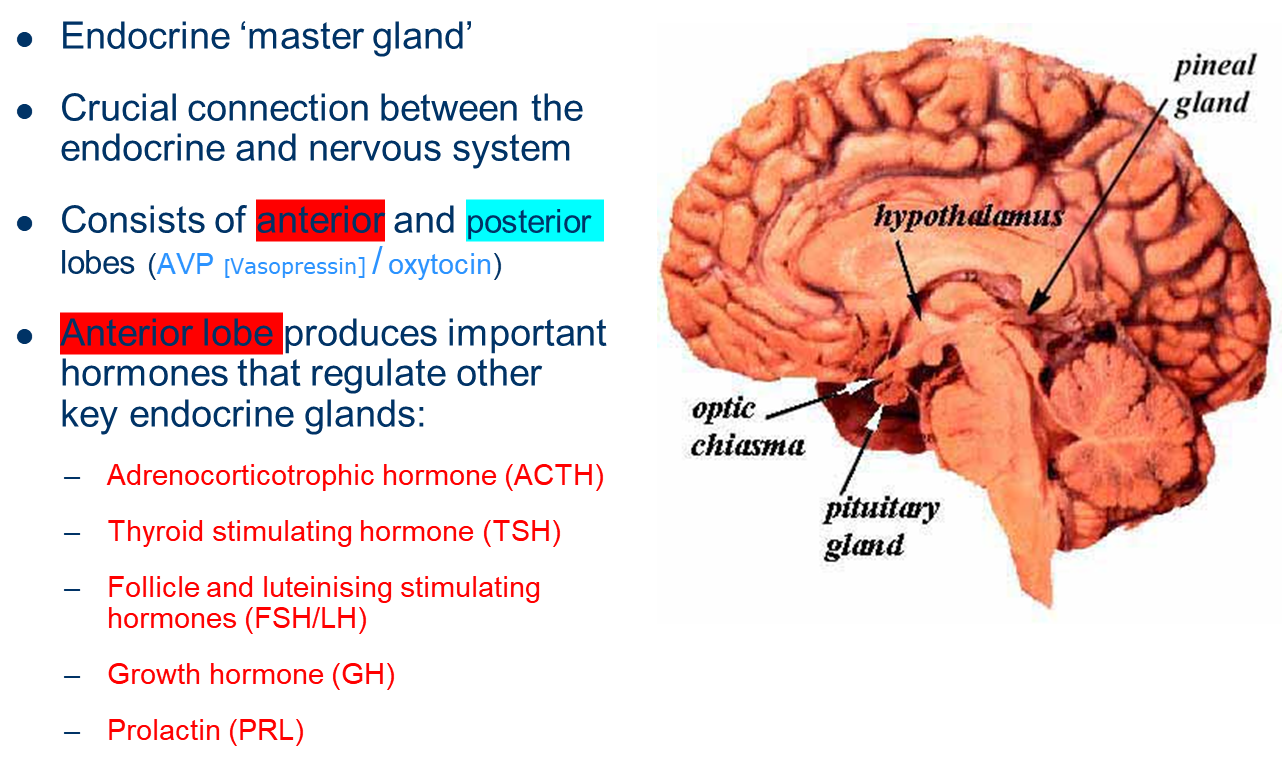
describe the endocrine gland: Pituitary
describe the endocrine gland: Thyroid
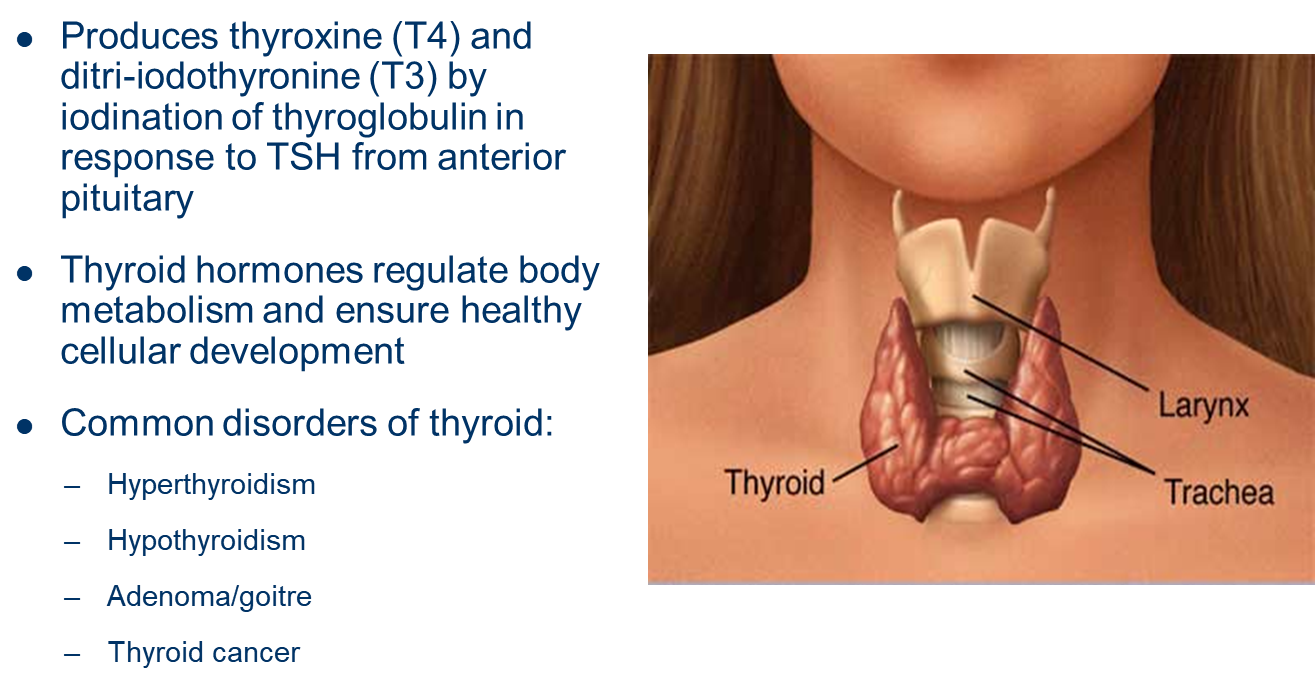
describe the endocrine gland: Adrenals
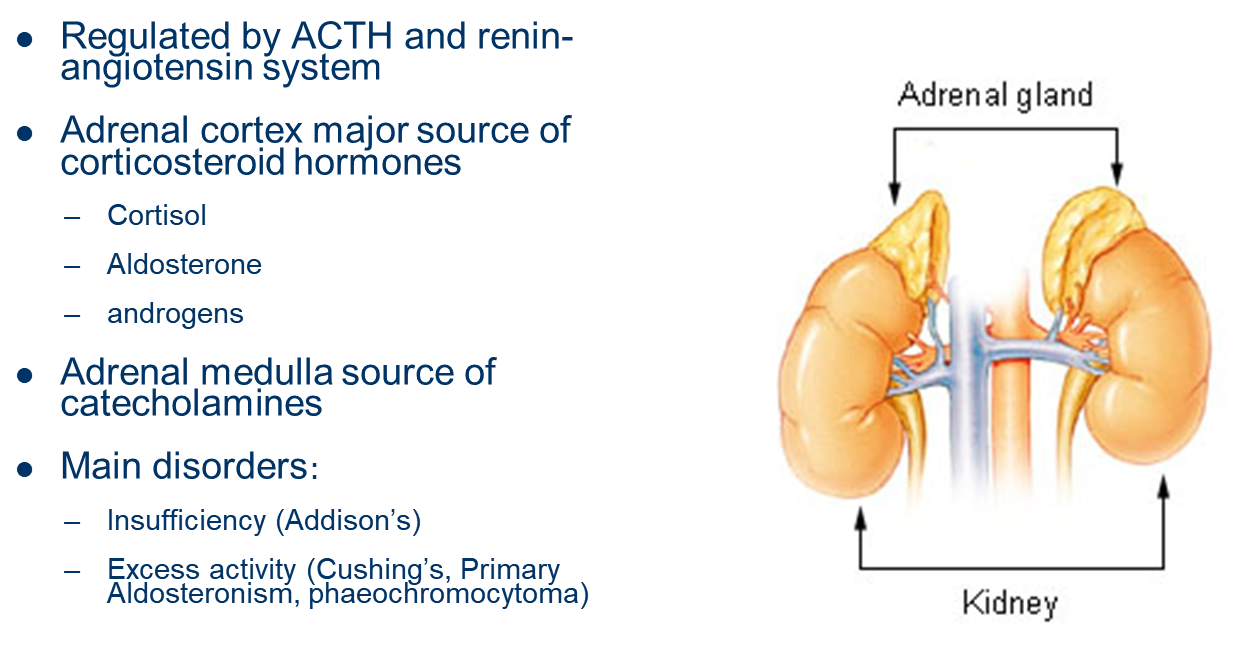
describe the endocrine gland: Gonads

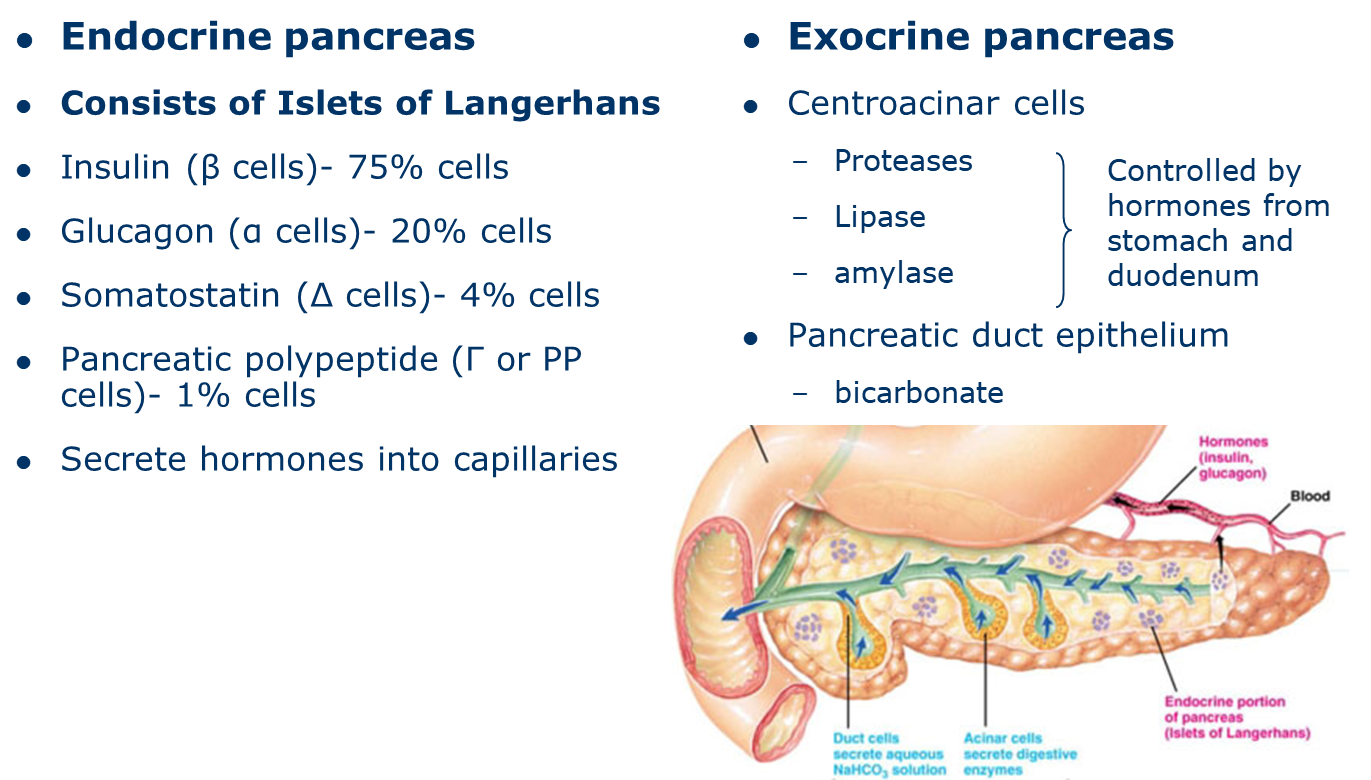
describe the endocrine gland: the pancreas
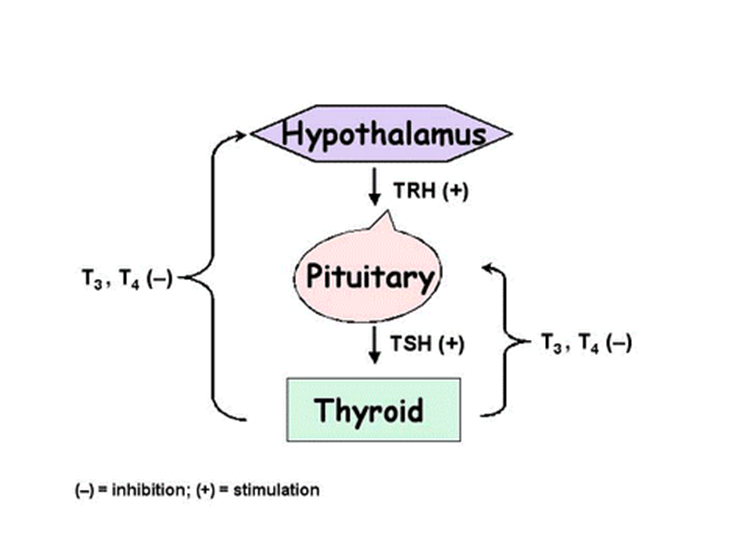
explain the 3 Feedback loops in endocrinology
all the feedback is to re-establish Homeostasis
PANCREAS FEEDBACK LOOP:

THYROID FEEDBACK LOOP:
HYPOTHALAMIC-PITUITARY-ADRENAL (HPA) AXIS:
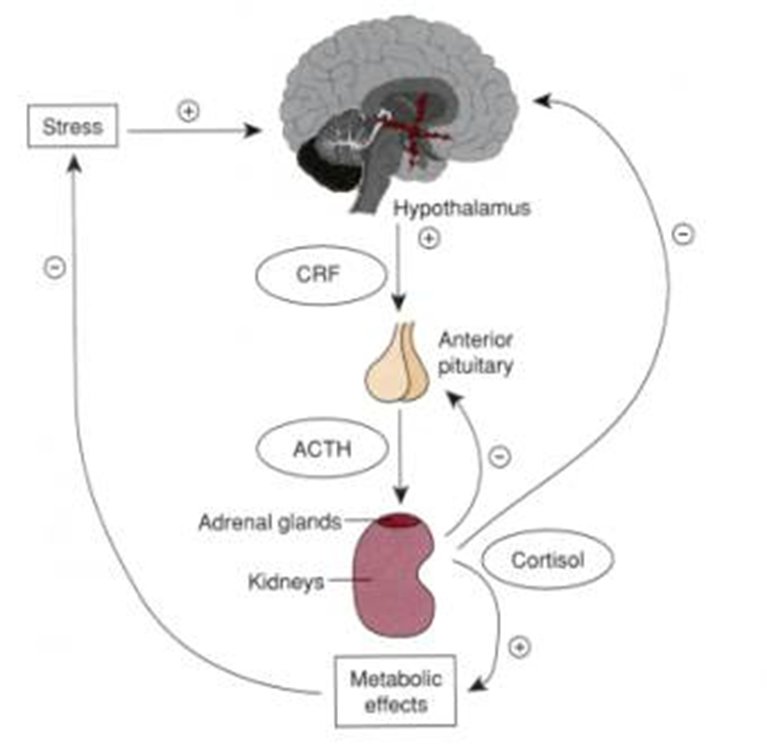
cortisol is a potent anti-inflammatory hormone
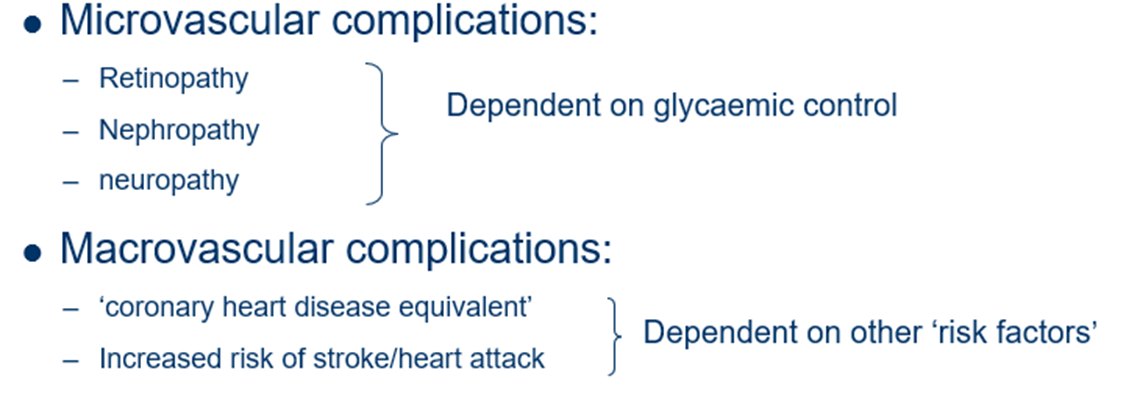
what are the microvascular and macrovascular complications of diabetes mellitus?
what hormonal/incretin obesity treatments are used?
GLP-1 analogues
Combined GLP-1 and GIP analogues
GLP-1 – Semaglutide (oral/subcutaneous) and dulaglutide (subcutaneous)
GLP-1/GIP – Tirzepatide (mounjaro) subcutaneous once weekly 5-15mg
Weight loss around 7-20% at 1 year
what cells are auto-destructed in Type 1 Diabetes?
one of the cell type in Islet of Langerhans =
β (beta) cells= which secrete insulin
why do islet of Langerhans have a good blood supply?
to detect blood sugar changes in the blood
why do insulin and glucagon have to be injected and cannot be taken orally?
they’re proteins- short peptide hormones.
You would digest them otherwise.
how is Insulin synthesised?
insulin is synthesised as a pro-hormone.
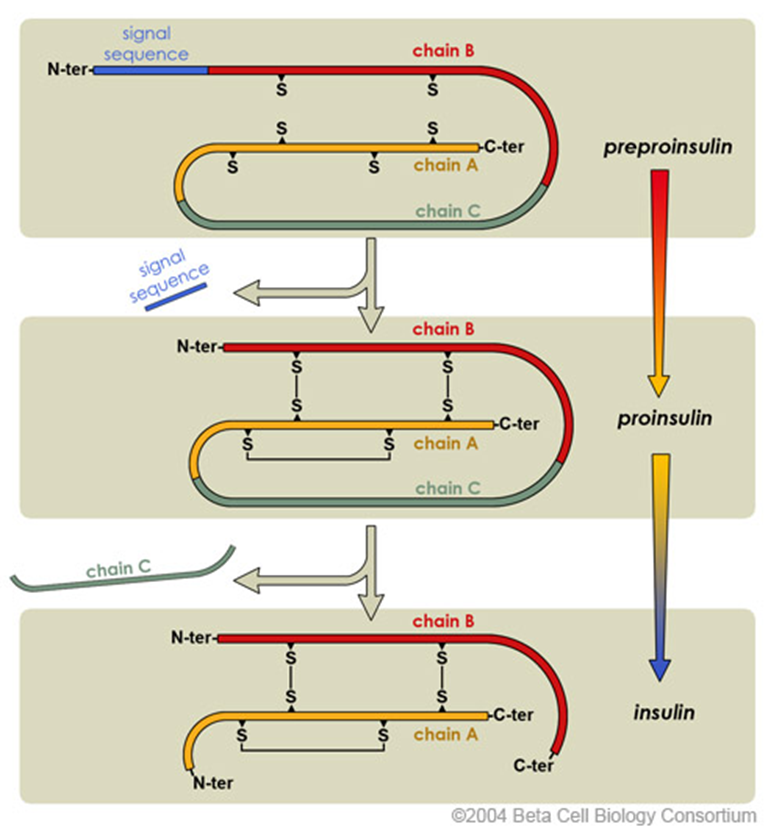
the N-terminal signal peptide is removed from Preproinsulin, this converts it into Proinsulin. to get from Proinsulin to insulin, the protein folds and forms disulfide bonds between cysteine amino acids. the C chain is also cleaved (but remains there).
S= sulfur - it’s part of the atom makeup of cysteine
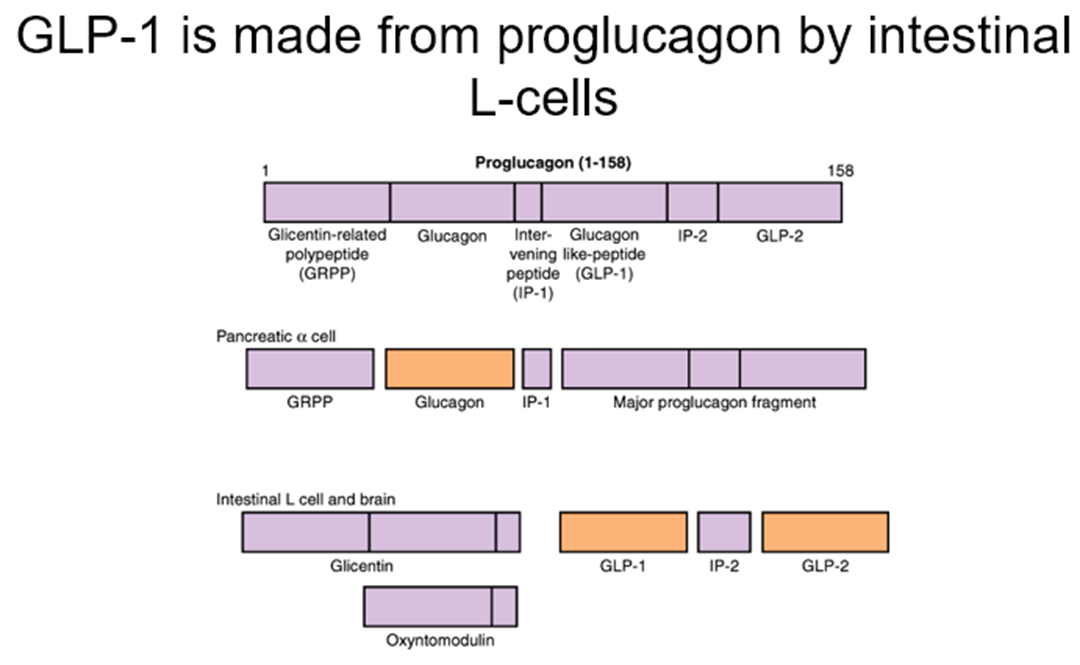
how is glucagon synthesised?
glucagon is synthesised as a pro-hormone.
it’s derived from Proglucagon.
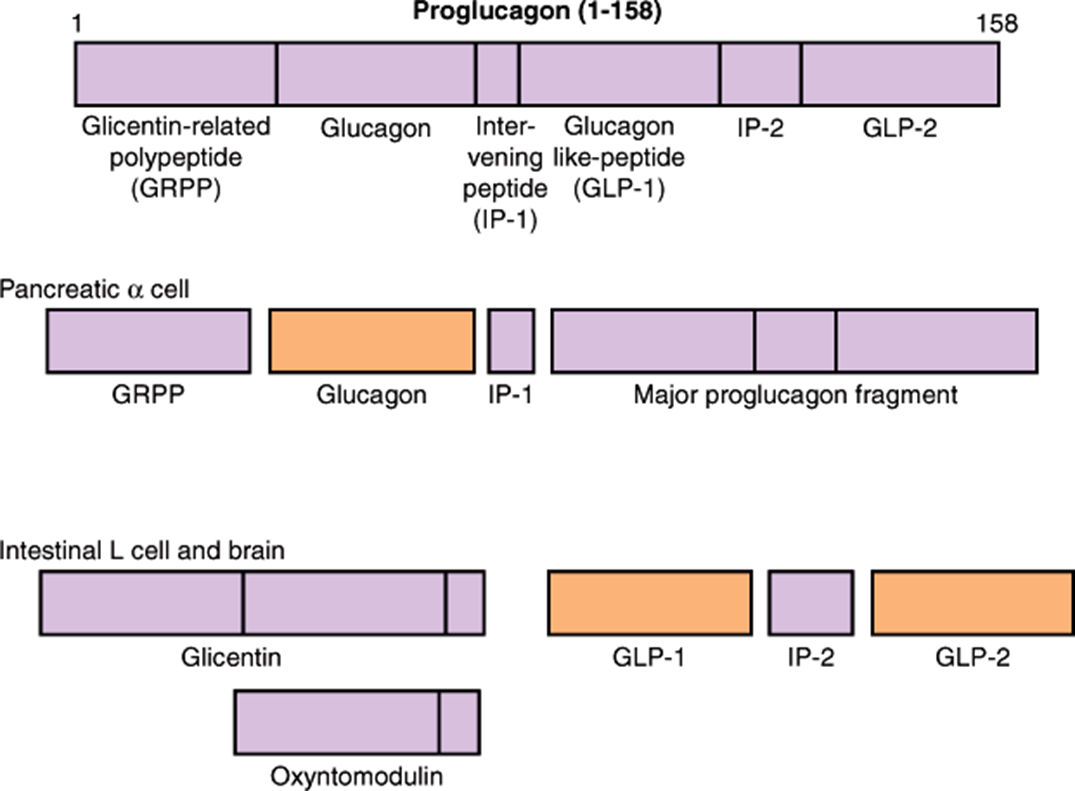
Proglucagon is cleaved to glucagon in α-cells and GLP in the gut/certain neurones
describe Hexokinase IV (glucokinase) and differentiate from Hexokinase I,II,III
beta cells (pancreas) contain glucokinase/hexokinase 4 which has low affinity for glucose
Hexokinase 1-3 have a high affinity for glucose.
Glucokinase is critical for glucose sensing by the β-cell
–Ensures rate of G6P production increases as extracellular glucose concentration rises (e.g. after a meal)
–β-cells use G6P principally for ATP production by glycolysis/TCA cycle
–ATP concentrations increase in β-cell when extracellular glucose concentration rises
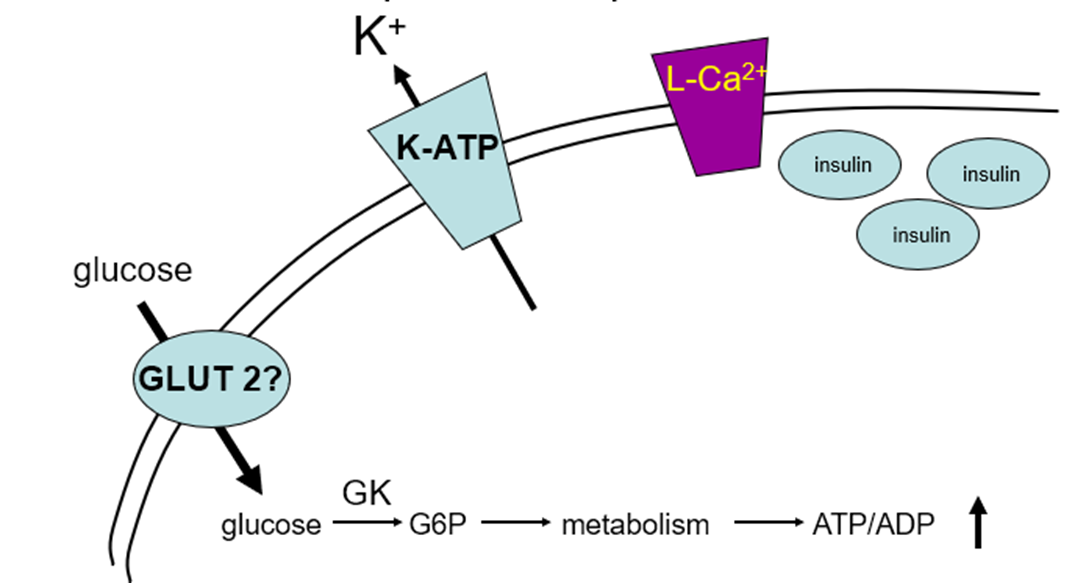
describe insulin secretion
insulin is a peptide hormone
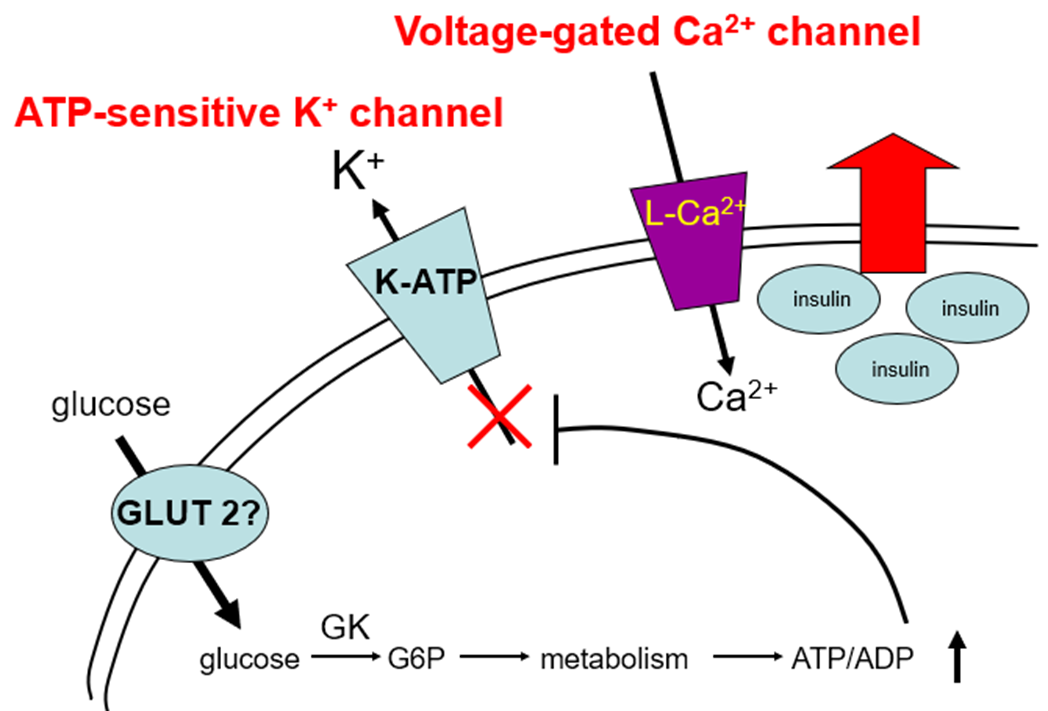
when ATP to ADP ratio increases, it acts as a signal and it closes ATP-sensitive K+ channels (which is found in the surface of beta cells and normally causes a potassium efflux). When they’re closed by ATP, K+ efflux stops and K+ stays in. This depolarises the membrane action potential of beta cells. This opens L type Voltage-gated Ca2+ channels, calcium floods in as the concentration outside the cell is high. Calcium acts as a signal for secretion, in beta cells what’s secreted is granules containing Insulin. mature Insulin will also contain C-peptide and is secreted by exocytosis, it will fuse to the membrane and release its contents.
When the glucose goes away, ATP levels go down, the K+ channel is not inhibited anymore, depolarisation is no longer happening so Voltage-gated Ca2+ channels are not open and insulin secretion stops.
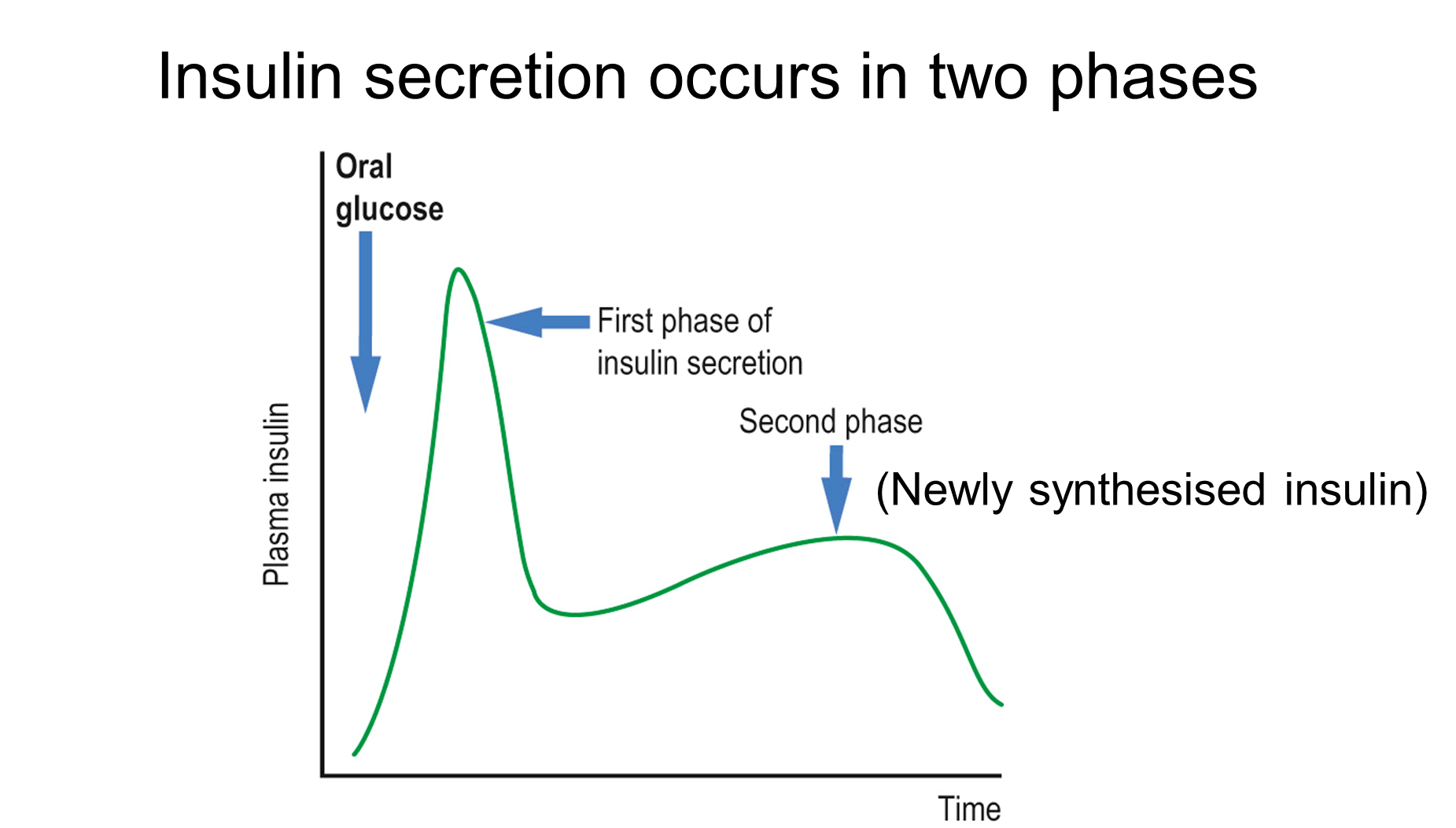
outline glucagon secretion
not well understood!
•Glucagon secreted in response to low [glucose]
•May be crosstalk between glucagon and insulin secretion in human islets (glucagon may inhibit insulin secretion and vice versa)
explain insulin signalling
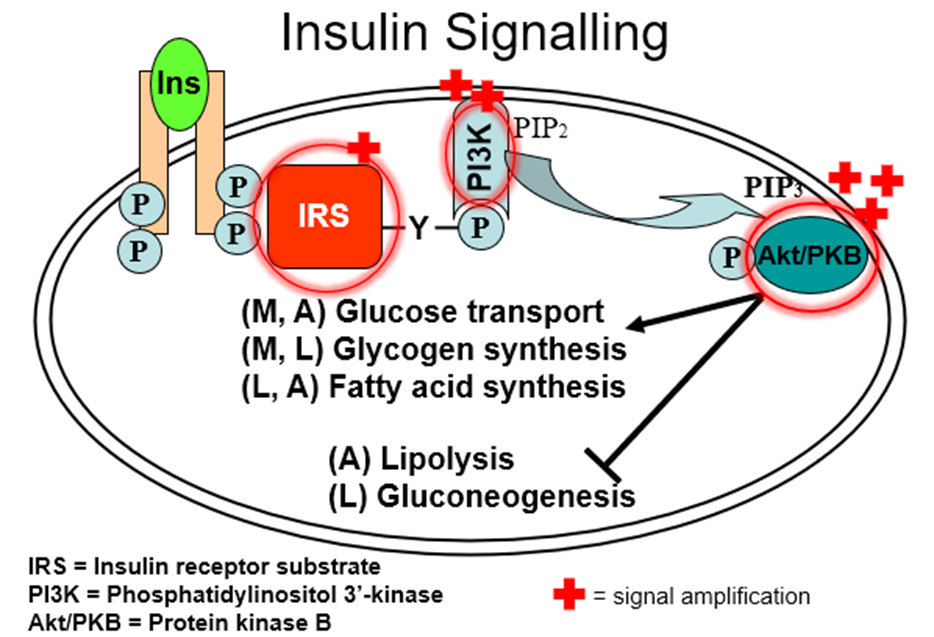


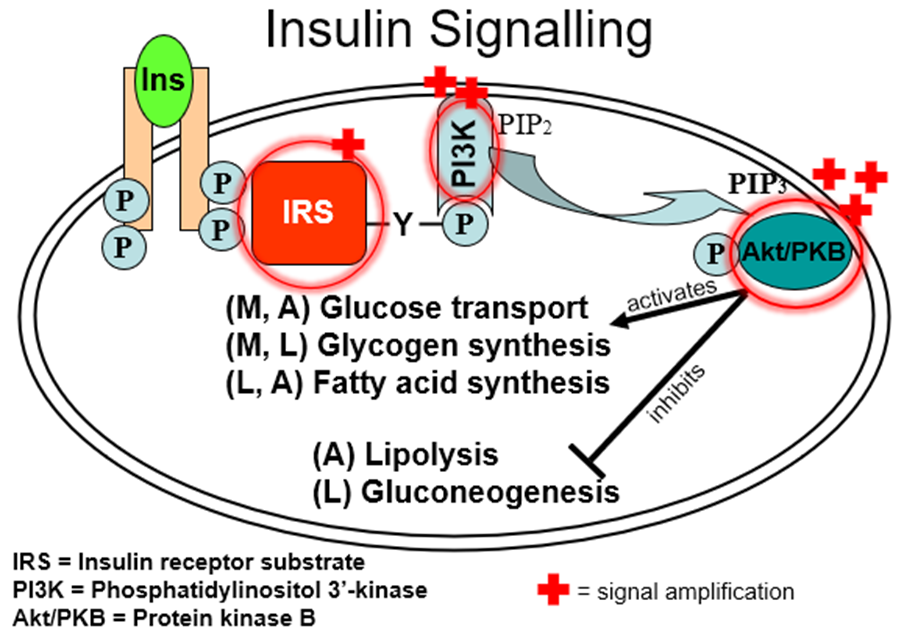
in what level of the insulin signalling process do people with type 2 diabetes struggle?
in the IRS (insulin receptor substrate) proteins
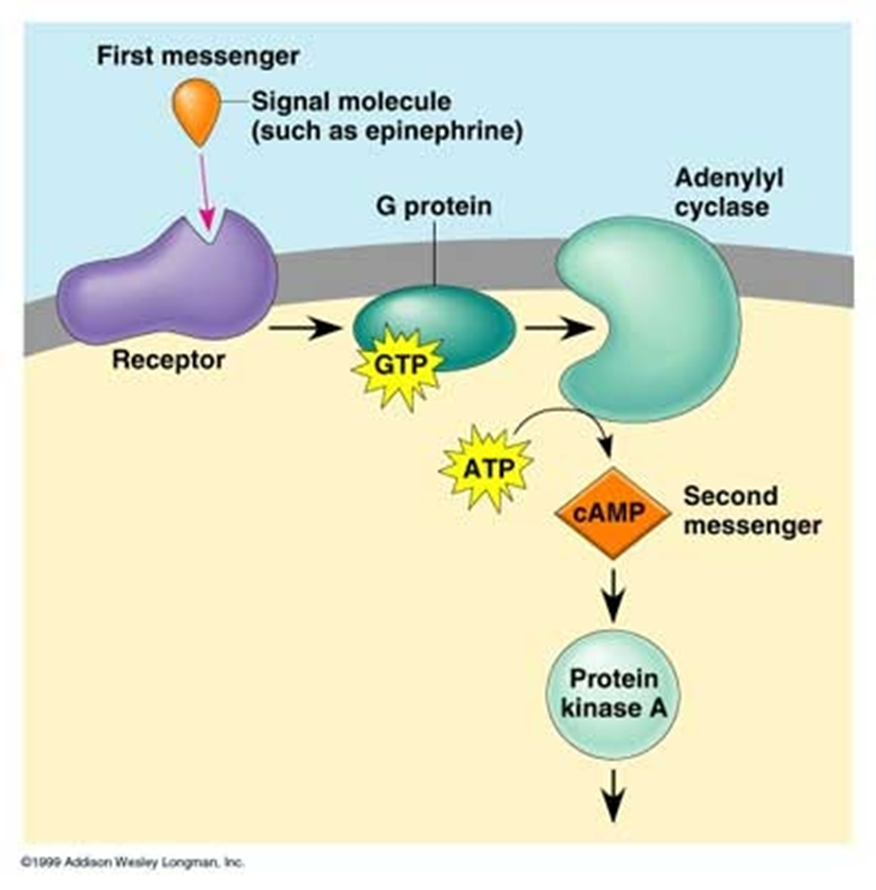
explain glucagon signalling
•Glucagon binds to the Glucagon Receptor
–G-protein coupled receptor (GPCR)
–Found in hepatocytes (therefore effects largely restricted to liver)
– Increases cyclic AMP (cAMP)
– cAMP stimulates cAMP-dependent protein kinase (PKA)

describe Carbohydrate homeostasis, and the action of insulin and glucagon on it
Carbohydrates are transported as glucose, stored as glycogen.
• Some tissues dependent on a constant supply of glucose (e.g. RBC, Brain/CNS)
• Plasma glucose concentration is tightly regulated:
Fasted 4-5 mM
After meal 8-12 mM
• Principal regulation by insulin and glucagon
• Glucose can be synthesised de novo by the liver to buffer plasma glucose

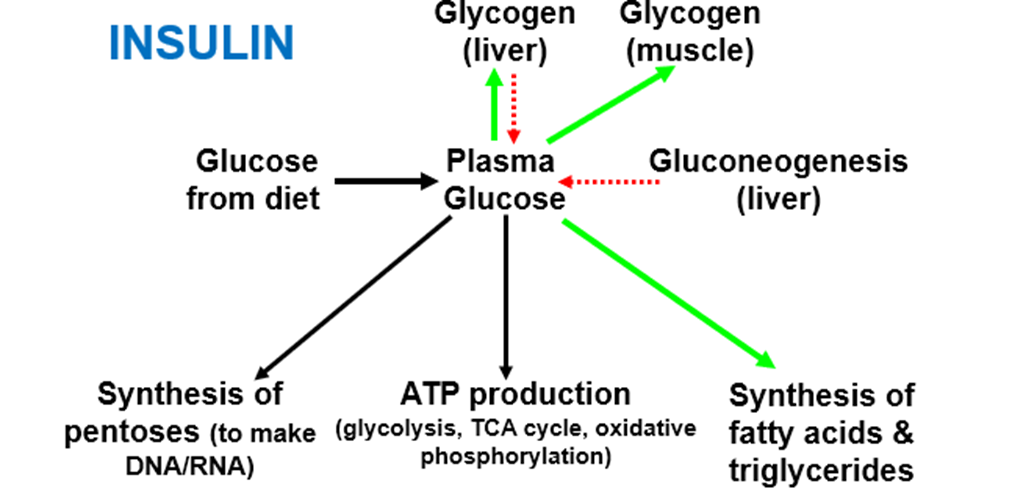

glucagon MAINLY acts on the LIVER
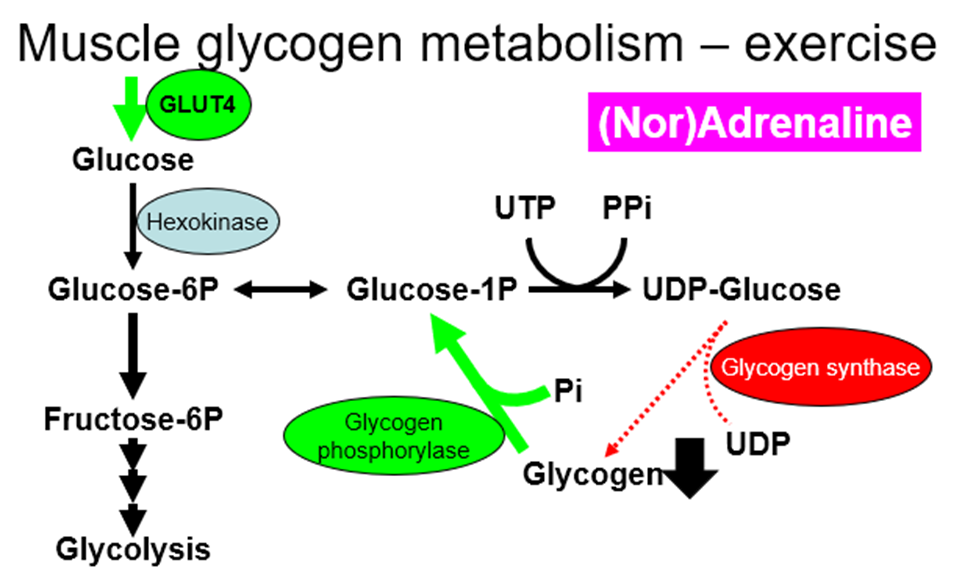
explain Regulation of glycogen synthesis AND glycogenolysis by hormones (insulin, glucagon, noradrenaline and adrenaline), in both a fed and fasted state/exercise, in both liver and muscle
glycogen
•Synthesized from glucose (Glycogen is a polymer of glucose)
•In liver
–storage for blood glucose maintenance
•In muscle
–storage for local energy production (only used by muscle itself)
liver:
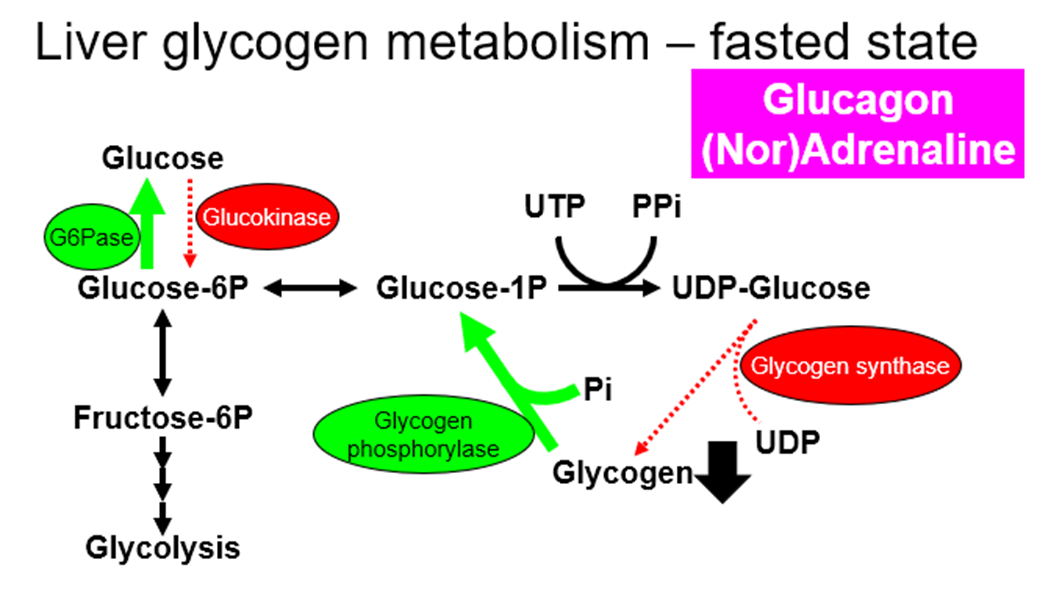
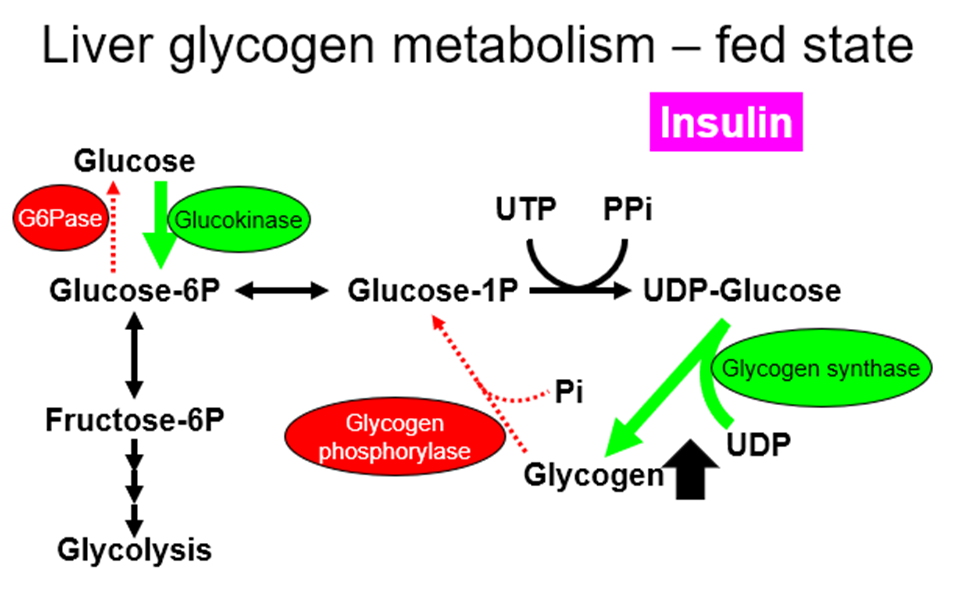
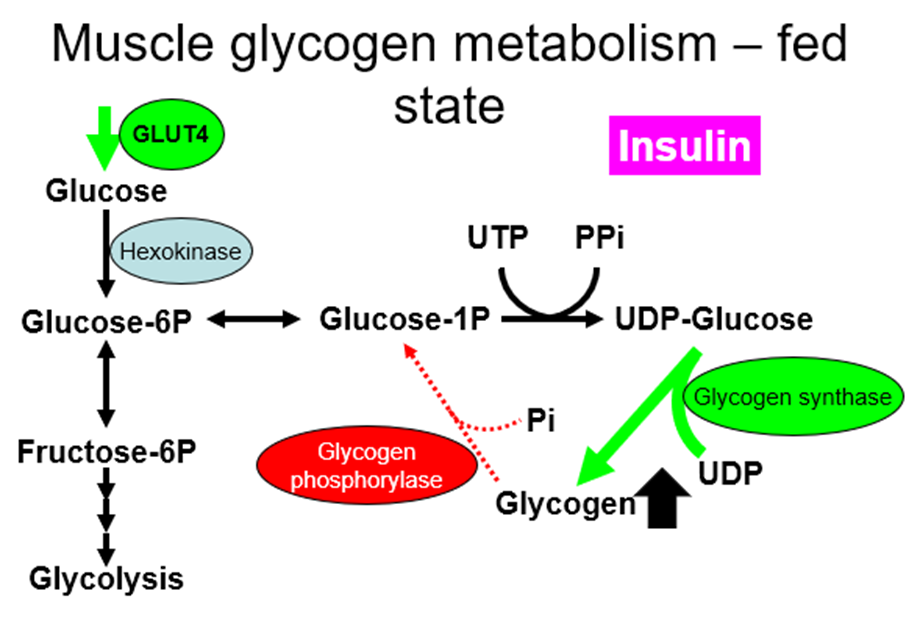
muscle:
Glycogen in muscles cannot go back to glucose, which is why only the liver glycogen can be used to maintain blood glucose levels
outline GLUT4
GLUT4 is insulin responsive (adipocytes & muscle only), which leads to:
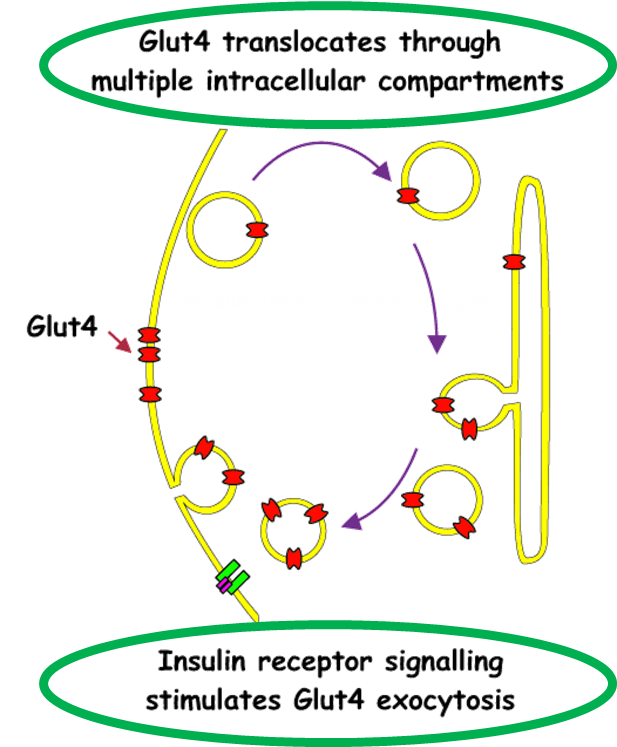
In adipose & muscle. Therefore, more glucose transported in (& converted to triglyceride or glycogen) when plasma [glucose] is raised after a meal.
GLUT4 is exercise responsive in muscle, in muscle, GLUT4 translocates in response to physical activity/exercise (independent of insulin) therefore, more glucose used for ATP production
outline gluconeogenesis
•The synthesis of glucose from a noncarbohydrate (nonhexose) source
–Lactate, Glycerol, Certain amino acids
•Occurs mainly in the liver
–Kidneys can contribute with prolonged starvation
•Essentially a reversal of glycolysis

Some steps are irreversible, and these steps need specific enzymes which are produced by the liver (indicated by the one-way arrows and enzymes written next to them)
describe hormonal regulation of gluconeogenesis
•Glucagon & Adrenaline
–Decreases Glucokinase activity (gene expression)
–Increases G6Pase and PEPCK activity (gene expression)
–Rapidly stimulates F1,6bPase and inhibits PFK and pyruvate kinase
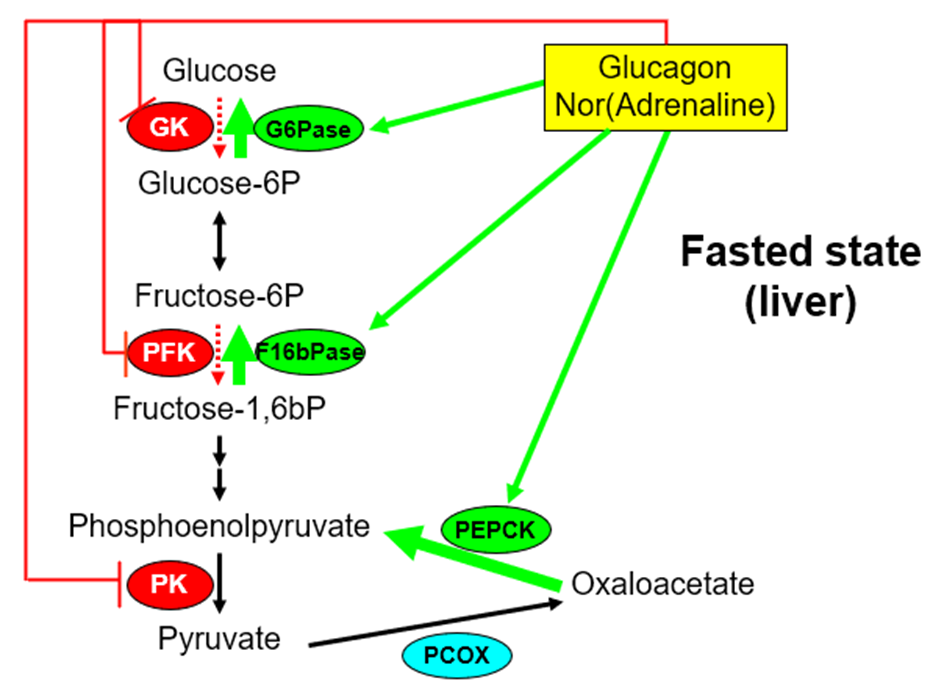
•Insulin
–Increases Glucokinase activity (gene expression)
–Decreases G6Pase and PEPCK activity (gene expression)
–Rapidly inhibits F1,6bPase and stimulates PFK
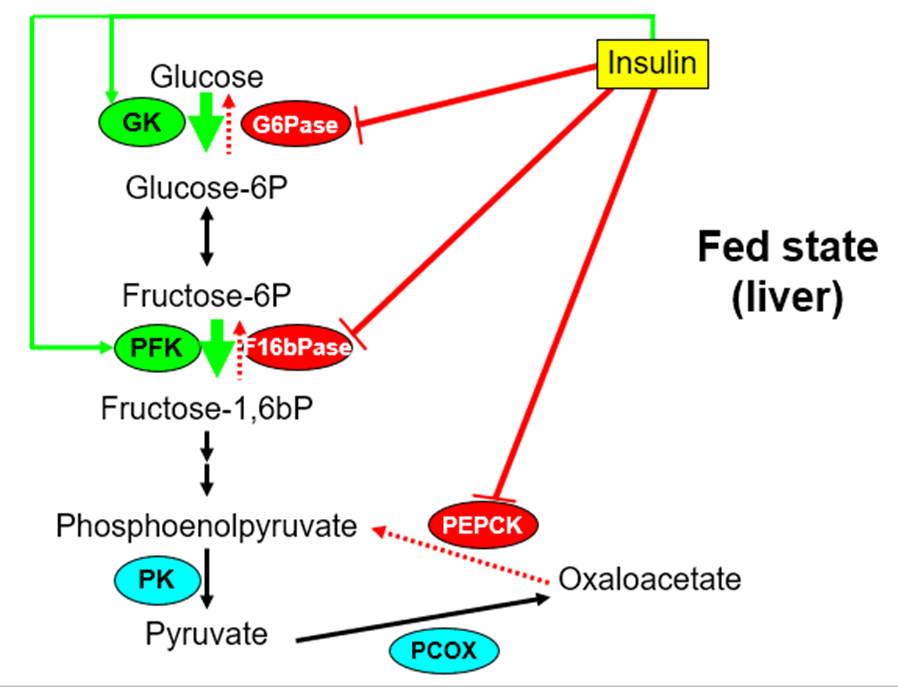
what are the Principal metabolic actions of Insulin?
•LIVER:
-Increases glycogen synthesis
-Increases fatty acid synthesis
-Inhibits gluconeogenesis (PEPCK & G6Pase)
•MUSCLE:
-Increases glucose transport (GLUT4)
-Increases glycogen synthesis
•ADIPOSE:
-Increases glucose transport (GLUT4)
-Suppresses lipolysis
-Increases fatty acid synthesis
Increases AA (amino acids) transport and protein synthesis in many tissues. Has specific effects on some genes.
outline Post-prandial (after a meal) metabolism
•Insulin inhibits adipose lipolysis
•Reduction in FA (fatty acids) concentration switches muscle to oxidation of glucose
•Insulin stimulates uptake of glucose by adipose and skeletal muscle (GLUT4)
•Glucose is used to make glycogen (muscle, liver), Triglycerides (adipose)
name the Hypoglycaemic drugs used for type 2 diabetes
•Sulphonylureas
•Biguanides
•Thiazolidinediones (NOT used anymore)
•Incretin mimics & DPP-4 inhibitors
•SGLT2 inhibitors
explain the hypoglycaemic drug group: Sulphonylureas, how it works and examples
inhibits ATP-sensitive K+ channels.
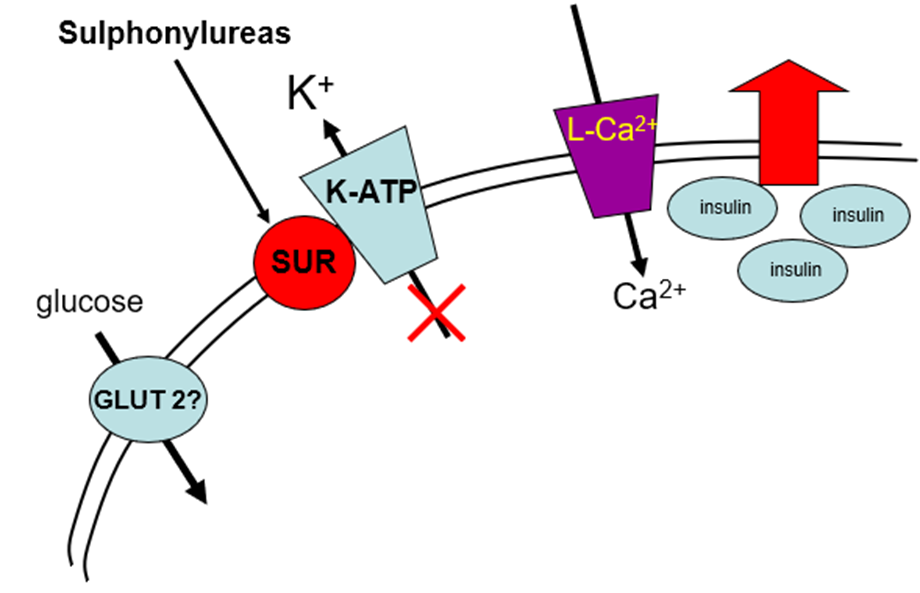
They attach to ATP-sensitive K+ channels, which leads to influx of Ca2+ and therefore release of insulin. They require some glucose to be present. They do not treat insulin resistance; they just allow more insulin to be released. They cannot be used in late type 2 diabetes because there’s a lack of beta cells to secrete the insulin.
•Glibenclamide, Gliclazide, Glimepiride, Tolbutamide, Glipizide used clinically
explain the hypoglycaemic drug group: Biguanides, how it works and the main example
Most widely prescribed hypoglycaemic drug, only drug of the Biguanides groups available= Metformin
•Mimics insulin by inhibiting hepatic gluconeogenesis
•Mechanism of action uncertain but all involve inhibition of liver mitochondrial function
•Increases AMP:ATP ratio which can suppress gluconeogenesis
AMP (adenosine monophosphate) has significant allosteric regulatory effects on key enzymes in glucogenesis (causes a conformational change that affects the active site switches of Phosphofructokinase-1 (PFK-1) and Fructose-1,6-bisphosphatase (FBPase)).
Can improve insulin sensitivity due to its ability to reduce liver fat
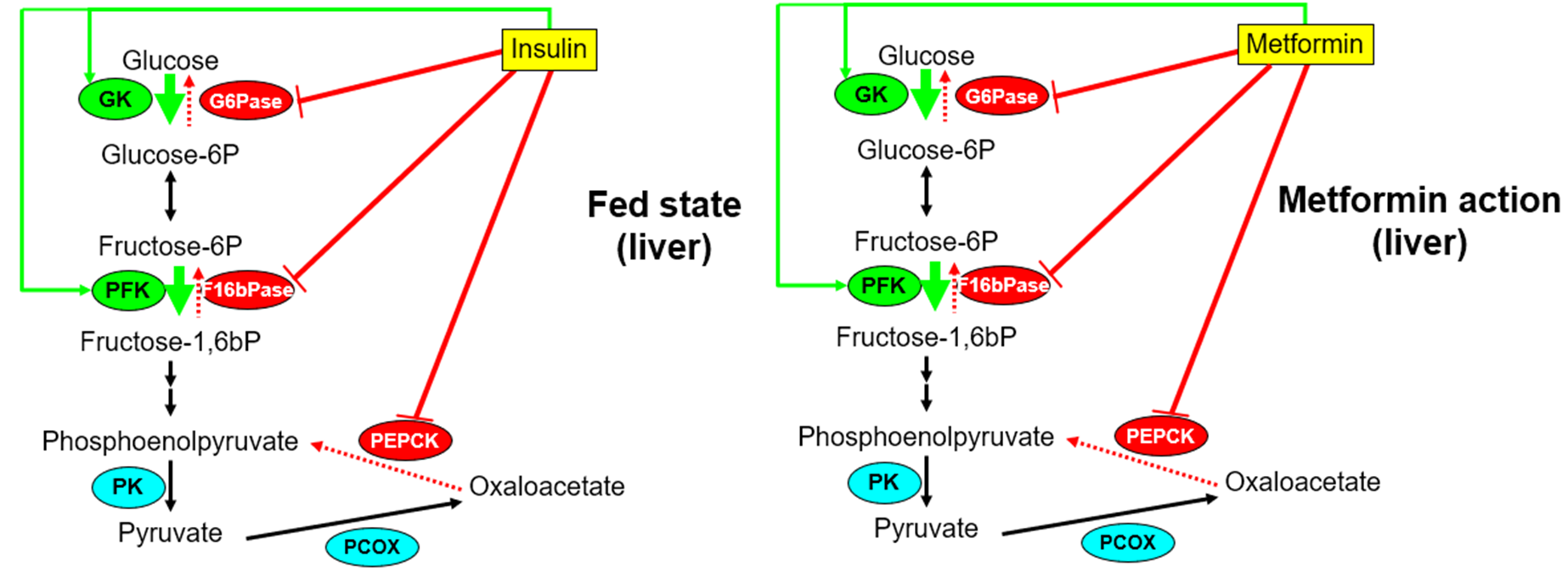
explain the hypoglycaemic drug group: incretin-mimetic (the incretin effect) AND DPP-4 inhibitors, how they work and examples
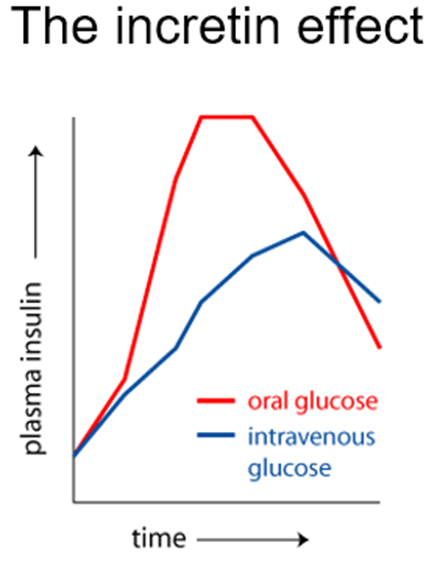
The incretin effect is the greater insulin response to oral versus intravenous glucose, a phenomenon caused by gut hormones called incretins.
Incretins
•Gastrointestinal hormones that potentiate glucose-stimulated insulin secretion
–Glucagon-like peptide-1 (GLP-1)
–Glucose-dependent insulinotropic polypeptide - also known as gastric inhibitory peptide (GIP).
–Rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4).
Incretin-mimetic mimics GLP-1 or GLP-1/GIP (only GIP not yet) OR they inhibit DPP-4, which allows endogenous Incretins to stat for longer in the body
Incretin mimetics
•Exenatide, Liraglutide, Semaglutide
–Mimic GLP-1, injected (peptide drugs)
–Modified so not cleaved by DPP-4
–Improve insulin secretion
–Reduce appetite and slows gastric emptying
–Semaglutide => Ozempic (for diabetes), Wegovy (for weight loss)
DPP-4 inhibitors
•Sitagliptin, Vildagliptin (2007)
–Inhibit DPP-4
–Increase endogenous incretin-mediated increase in insulin secretion
–Oral drugs
explain the hypoglycaemic drug group: dual incretin mimetic (Twincretins)
•Dual glucose-dependent insulinotropic polypeptides (GIP/GLP-1) have been recently developed.
•Tirzepatide (Mounjaro) is a unimolecular dual GIP/GLP-1 receptor agonist used as once weekly subcutaneous injection in T2D
•Improve glycaemic control and reduce body weight
explain the hypoglycaemic drug group: SGLT-2 inhibitors, how it works and examples
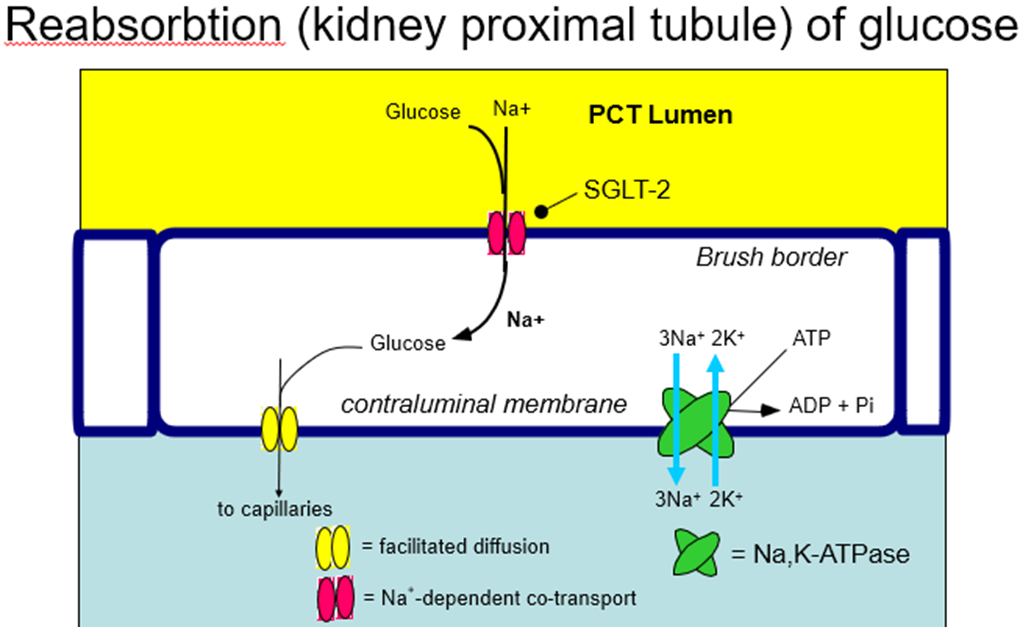
SGLT-2 reabsorb glucose into the bloodstream to prevent too much from being excreted/wasted. In someone with high blood glucose that’s positive, so these drugs inhibit SGLT-2 from reabsorbing all the glucose, decreasing blood glucose levels
SGLT2 Inhibitors
•Canagliflozin, Dapagliflozin, Empagliflozin
•Inhibit renal re-uptake of glucose from filtrate by SGLT2
•Reduces hyperglycaemia as glucose lost in urine
•Reduce heart failure and cardiovascular deaths in cohorts of heart failure, T2DM and chronic kidney disease
–effects appear consistent in patients with varying combinations of these diseases
–Mechanism by which they reduce CV deaths uncertain
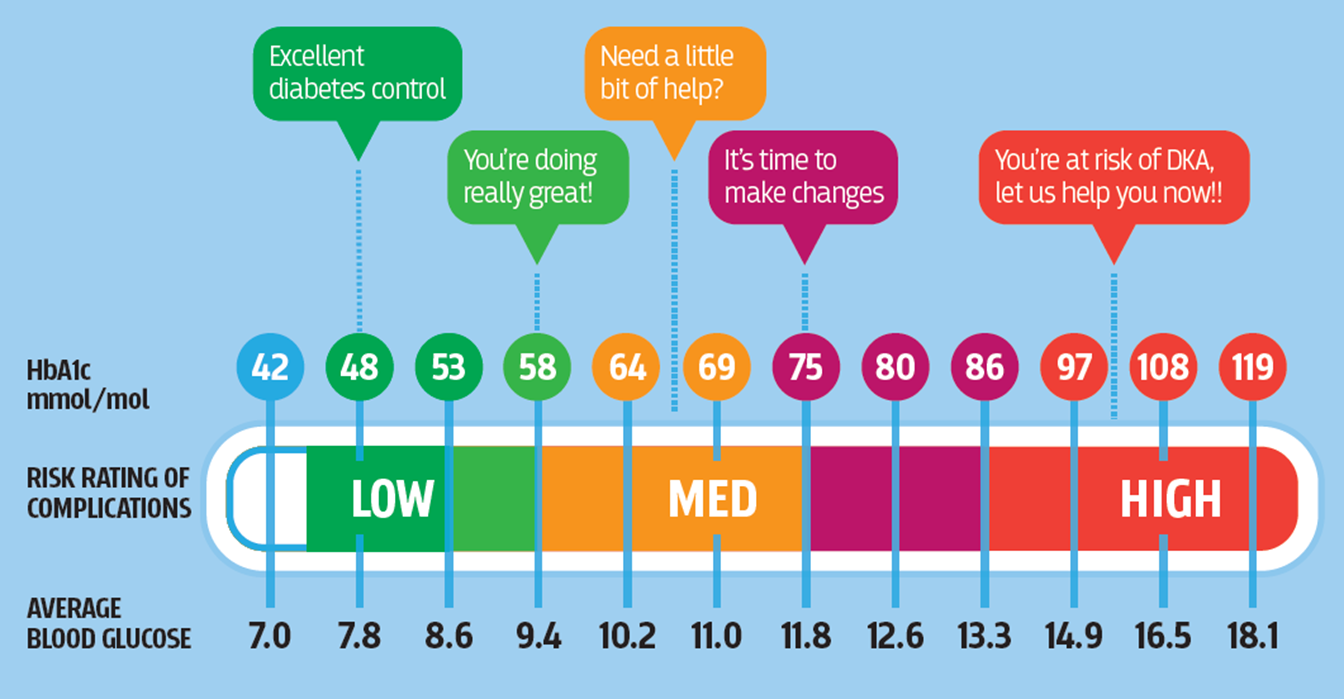
what’s HbA1c and what does it tell us?
name the Complications of T1DM
•Acute
–Ketoacidosis
–Hypoglycaemia
•Chronic
–Microvascular
•Retinopathy
•Neuropathy
•Nephropathy
–Macrovascular
•Ischaemic heart disease
•Peripheral vascular disease
•Cerebrovascular disease
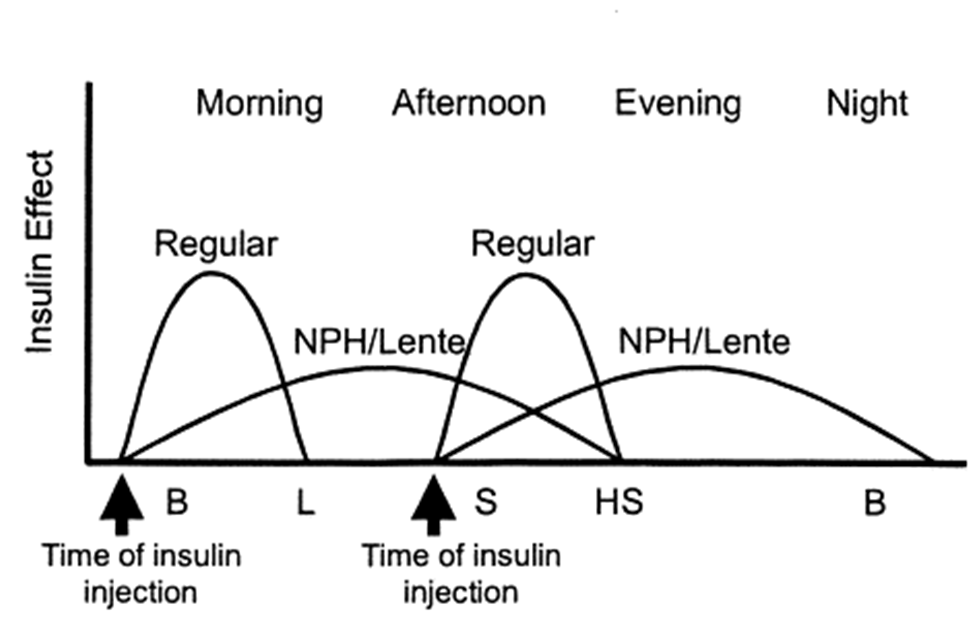
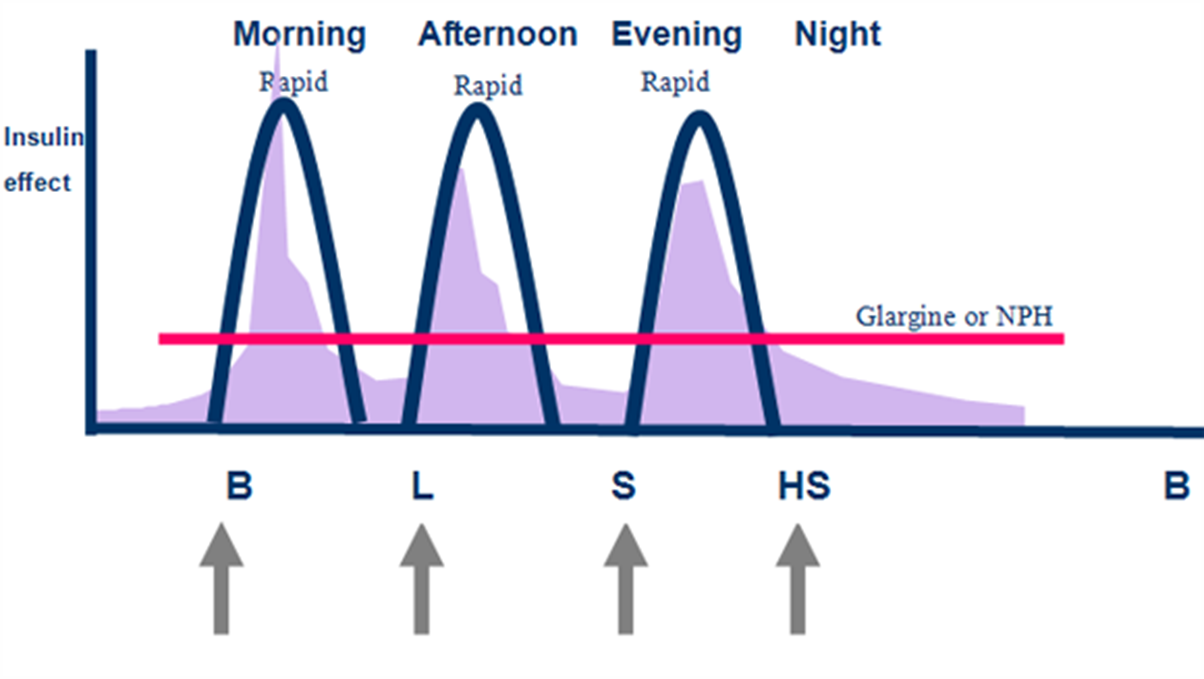
what’s the difference between Conventional and intensive regime of insulin?
Conventional regime (mix)
2 injections / day
Intensive regime (basal bolus)
4 injections / day
describe pathogenesis of T1DM
Capillary damage
•Hyperglycaemia
–Structurally and functionally abnormal small vessels
•Increased capillary pressure
•Thickened and damaged vessel walls
•Endothelial damage
–Leakage of albumin and other proteins
Metabolic damage
•Most tissues need insulin to take up glucose
–Retina / kidney / nerves don’t
•Glucose metabolised to sorbitol by aldose reductase
If [glu] rises
•Excessive glucose enters polyol pathway
–Sorbitol accumulates
–Less NADPH available for cell metabolism
– build up of ROS and oxidative stress
–Cell damage
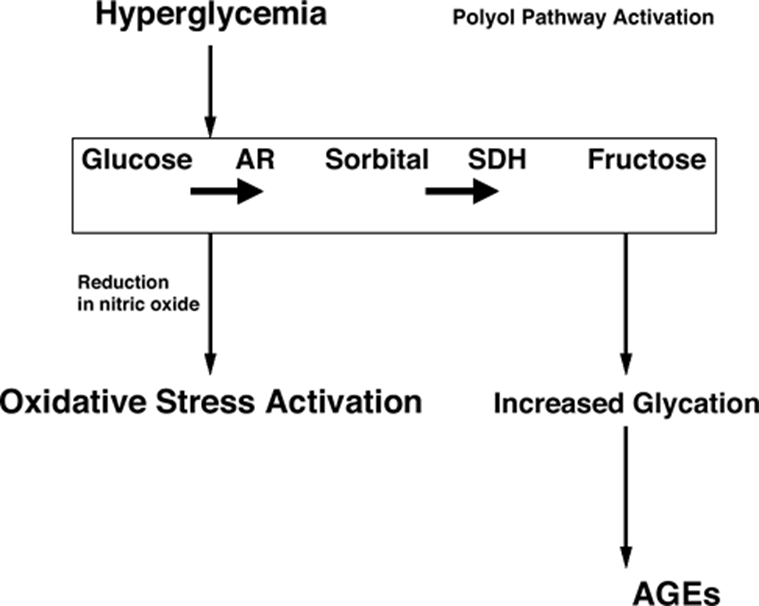
explain Diabetic retinopathy
•The hallmark of diabetic complications
•No symptoms until it’s too late
•Leading cause of impaired vision in aged 20-74
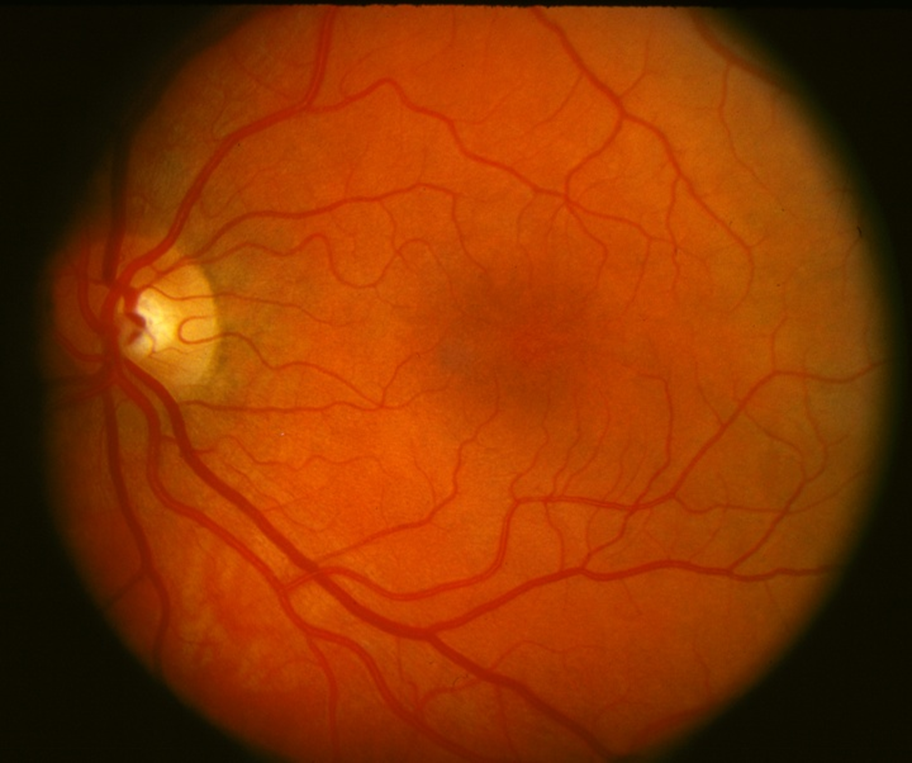
Early stages (non-proliferative)
•Hyperglycaemia
–Damage to small vessel wall
–Microaneurysms
•When vessel wall is breached
–Dot haemorrhages
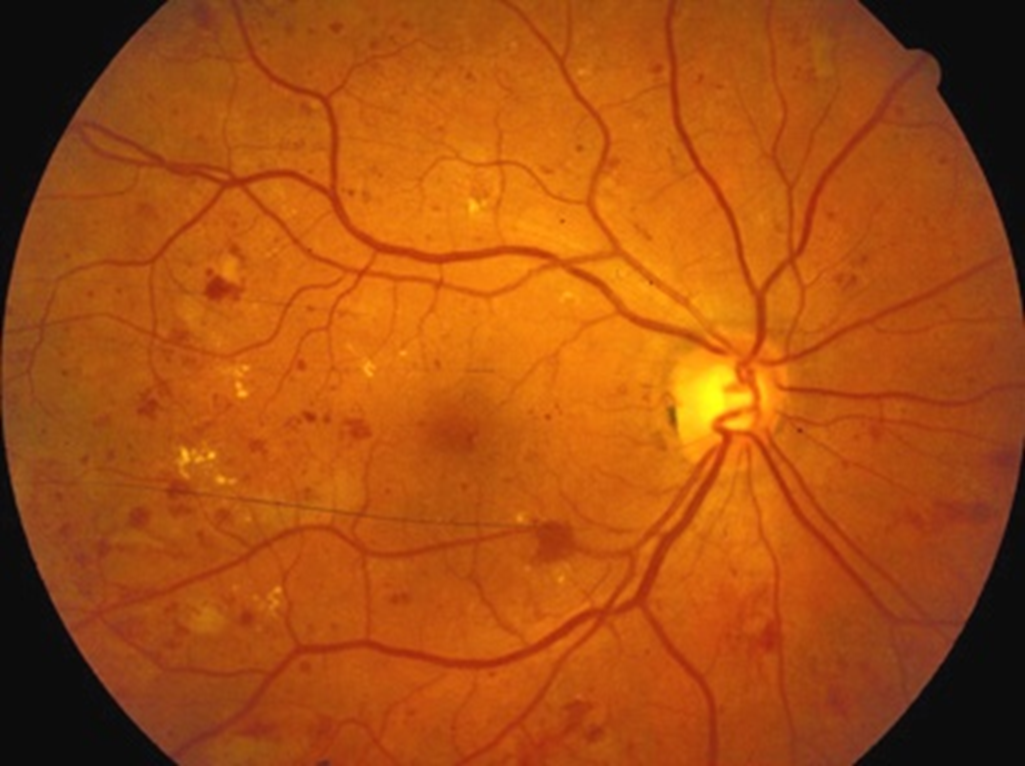
•Protein and fluid left behind
–Hard exudates
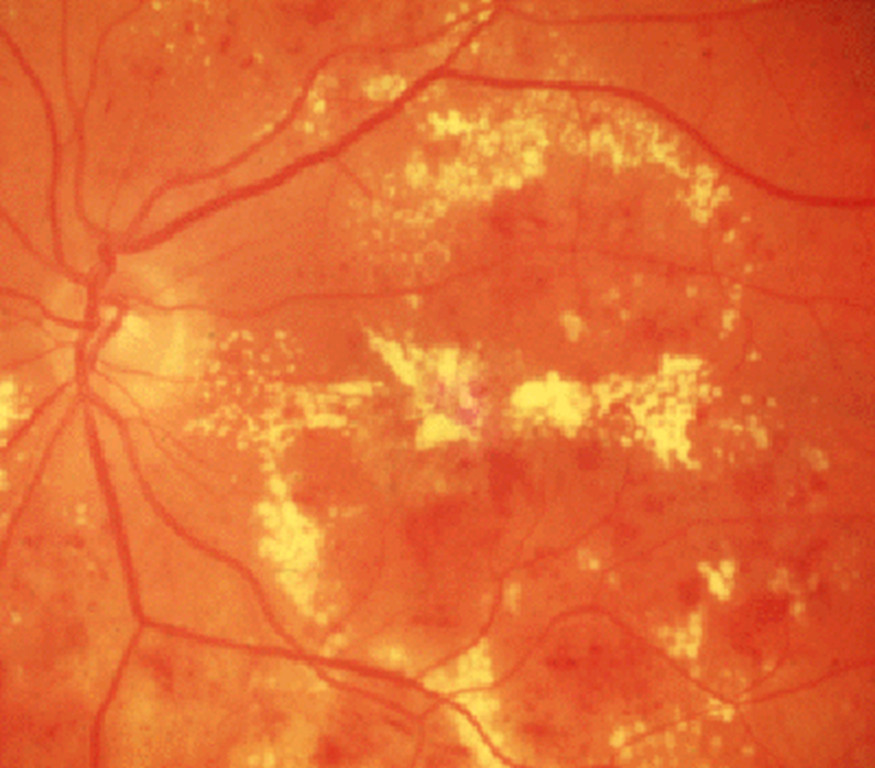
•Micro-infarcts
–Cotton wool spots
Later stages
•Damage to veins
–Venous budding
–Blockage of blood supply
•Ischaemia→ VEGF and other growth factors
–Neovascularisation
–Proliferative retinopathy
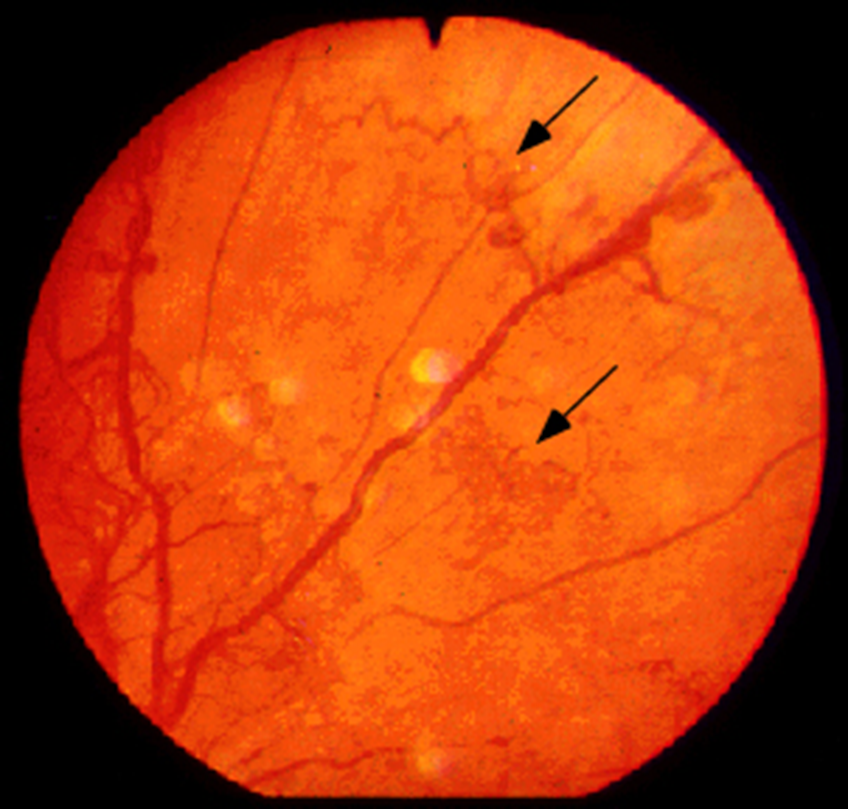
–Vitreous haemorrhage
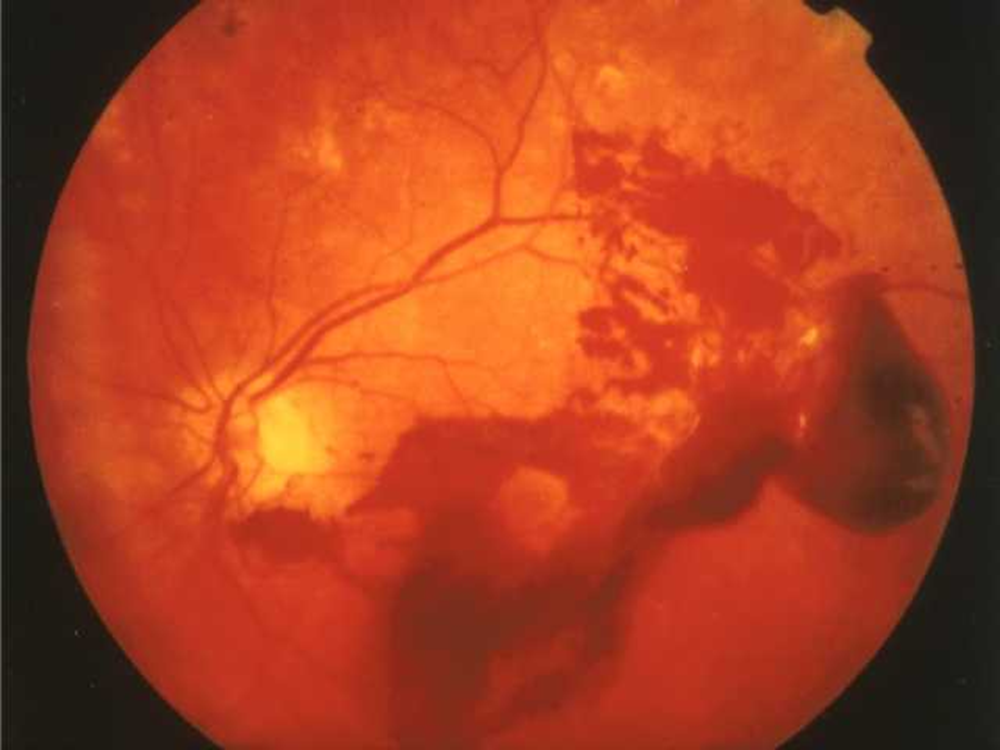
•Fluid not cleared from macular area
–Macular oedema
what’s the prevention and treatment of retinopathy?
•Good glycaemia control
•Stop smoking
•Good blood pressure control
Retinal Screening:
•Annual from age of 12 yrs
•Refer to ophthalmology if sight threatening
•Treatment
–Address risk factors
–Ophthalmic review
•laser
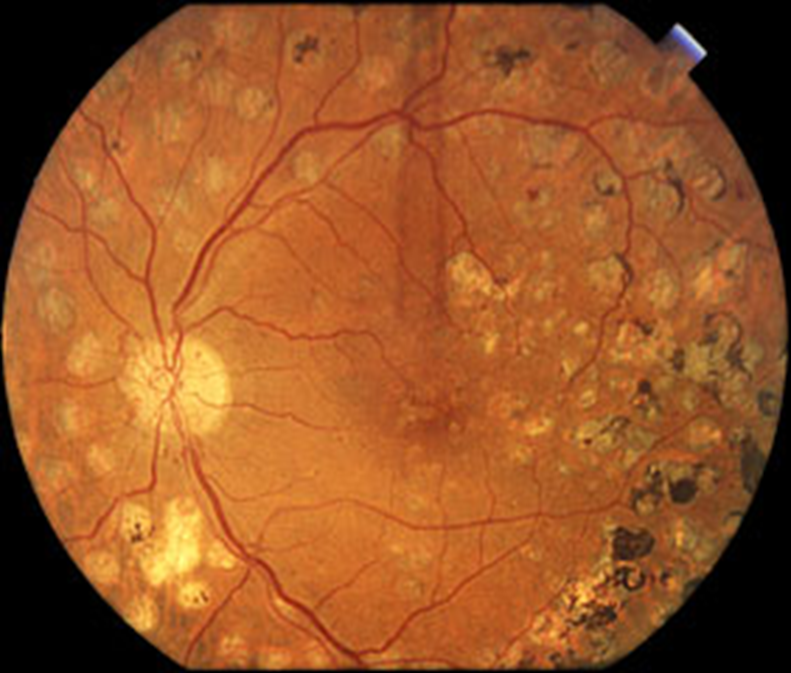
•VEGF inhibitors (bevacizumab)
•vitrectomy
describe Diabetic nephropathy
Stages of diabetic nephropathy:
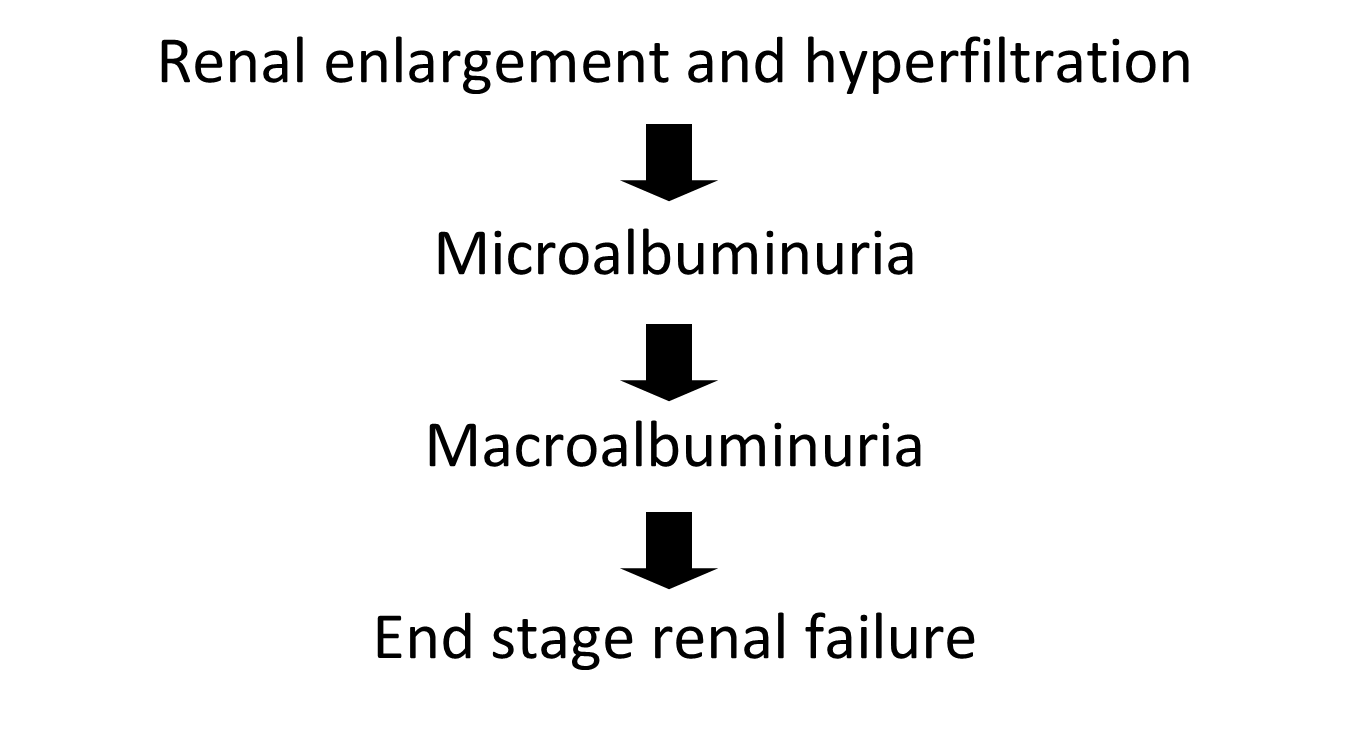
Microalbuminuria
•Tiny traces of albumin
–Too small to be detected on Dipstick
–30-300 mg albumin/24 hrs (normal <20)
–ACR >3.5 mg/mmol
•An independent CV disease predictor
•A unique window of opportunity
Pathophysiology:
first steps=>
•Renal hypertrophy
•Increase in GFR
•Afferent arteriole vasodilates
–Increased glomerular pressure
–thickened GBM
–capillary damage
–shear stress on endothelial cells
•End result- leakage of protein into urine
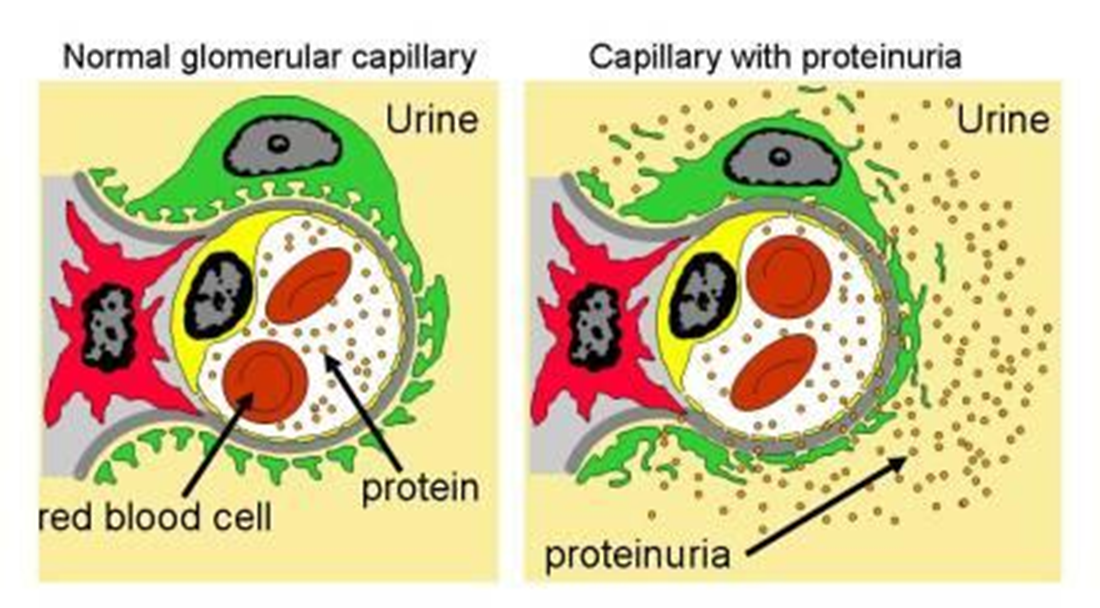
Later steps=>
•Progressive glomerulosclerosis
•Glomeruli destroyed
•Progressive proteinuria
–Nephrotic range
•Renal failure
what’s the prevention and treatment for Diabetic nephropathy?
•Screen for microalbuminuria
–Every year from diagnosis
•ACEI / ARB
–If microalbuminuria present
–Help to prevent progression to macroalbuminuria
•Aggressive CV risk reduction
–BP <125/75
–Statin
–Smoking
•Improve glycaemic control
–<7%
•Refer to renal clinic
–Once develop CKD
–ie eGFR<30
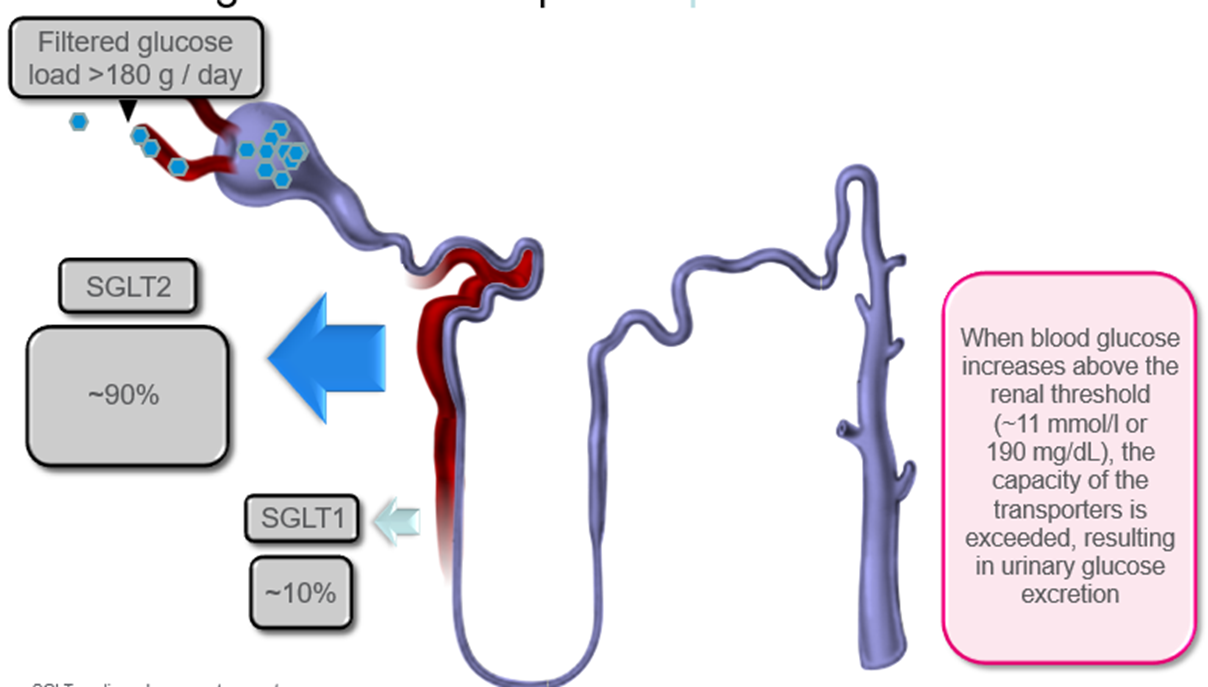
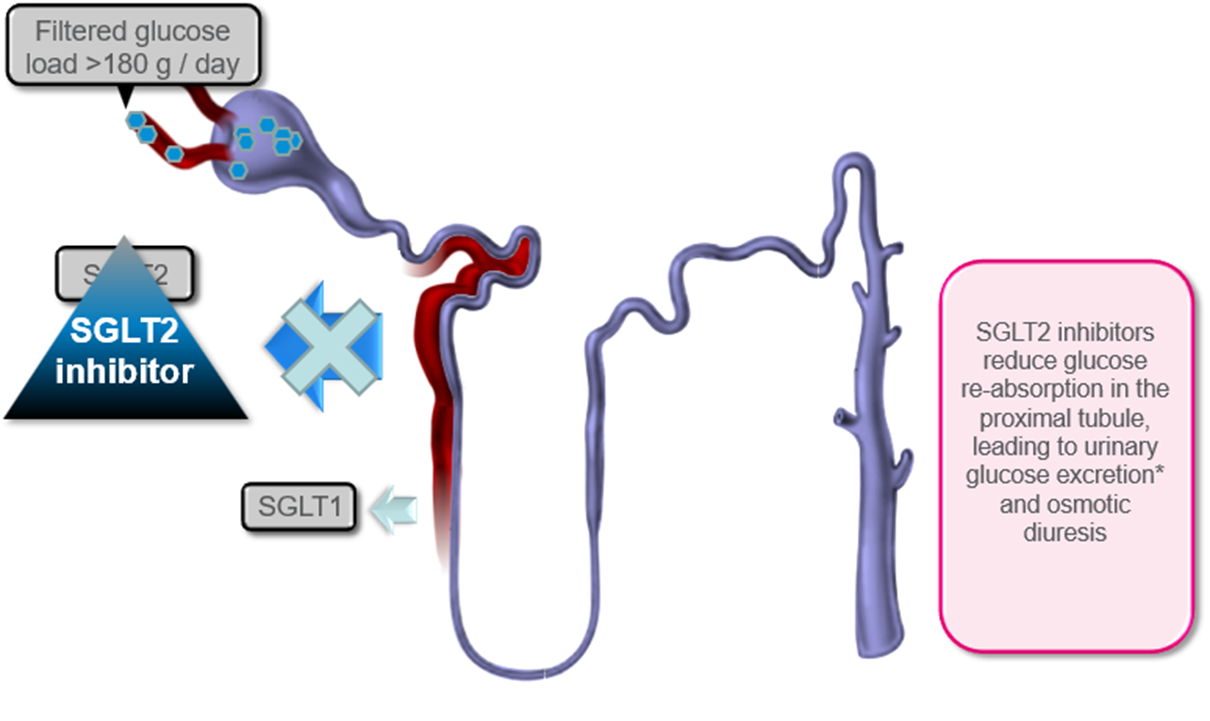
outline Renal glucose re-absorption in patients with diabetes and Urinary glucose excretion via SGLT2 inhibition
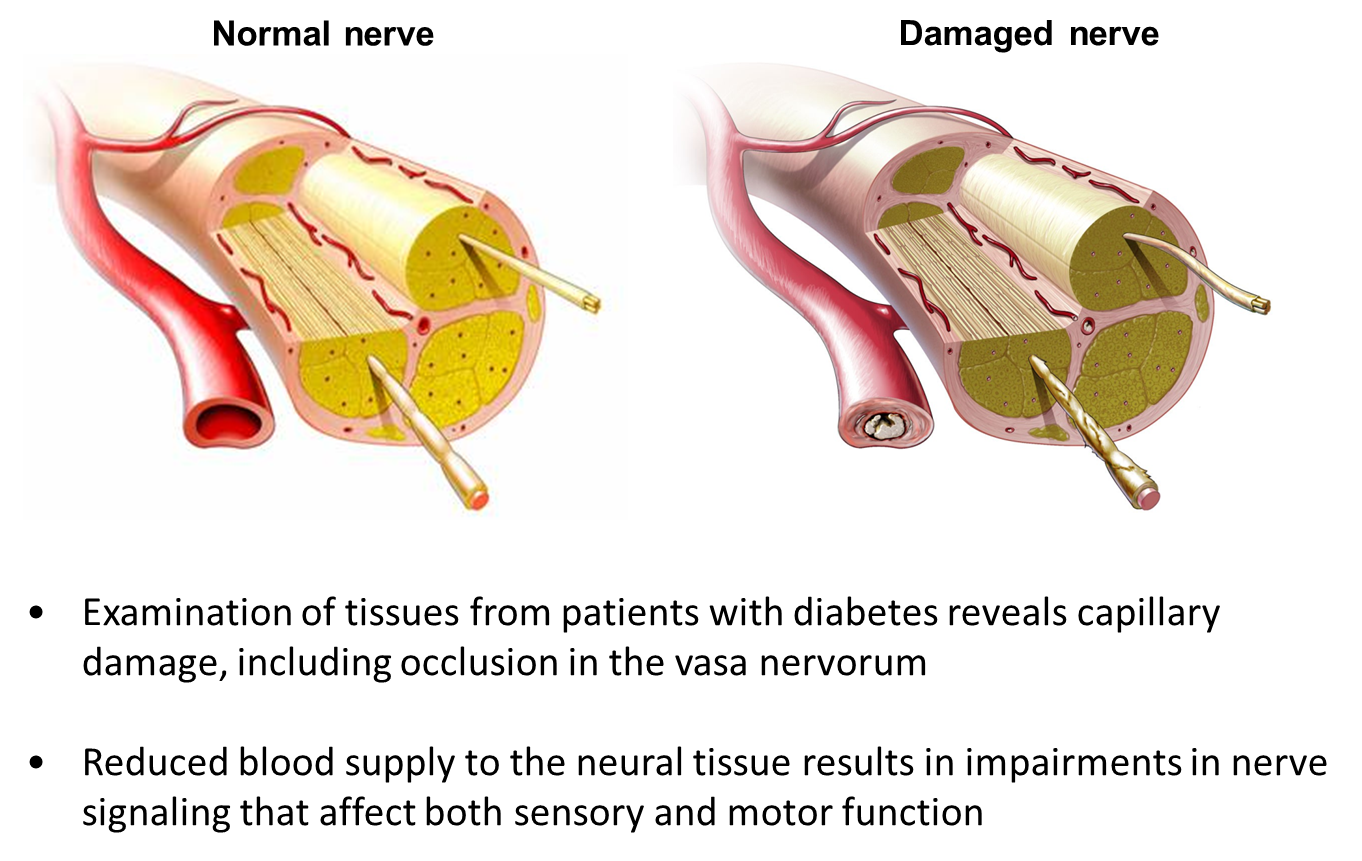
describe Diabetic neuropathy
•Peripheral (sensory) neuropathy
Diabetic foot
Charcot foot
•Autonomic neuropathy
•Cardiovascular
–Postural hypotension
•GU
–Erectile dysfunction
•GI
–Gustatory sweating
–Gastroparesis
•Mononeuritis multiplex
•Diabetic amyotrophy
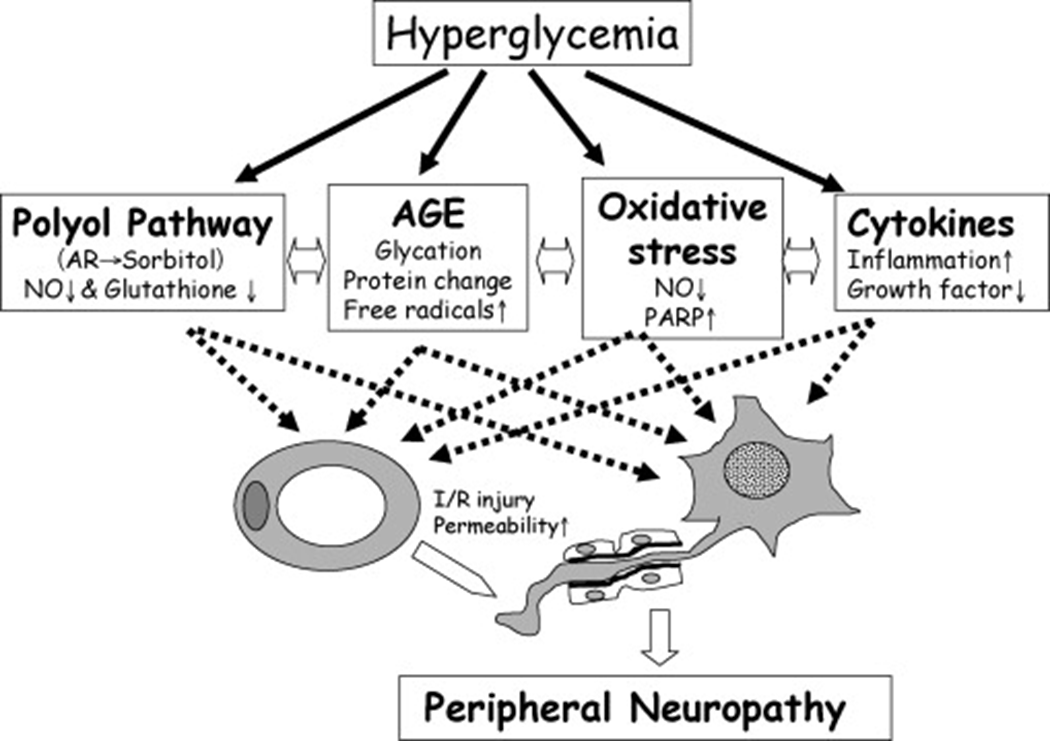

Neuropathy Testing
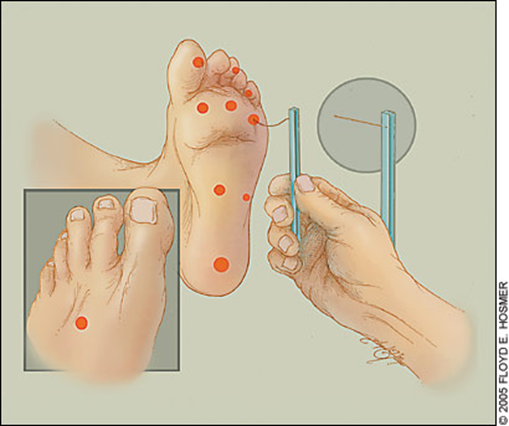
what’s Diabetic foot, Charcot foot?
Diabetic foot
•Combination of neuropathy and PVD (Peripheral vascular disease)
–Infection
–Ulcers
–Ischaemia
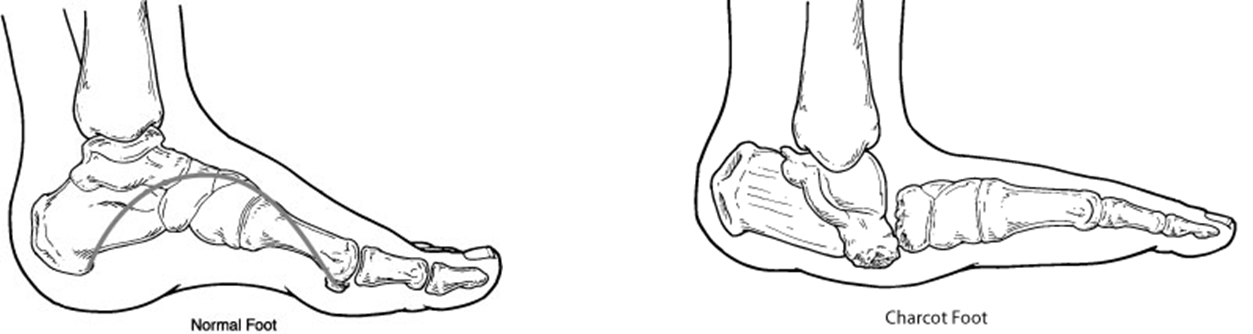
Charcot foot
•Numb foot
–Repetitive microtrauma
–Stress fractures
•Dysregulated blood flow
–Increased bone turnover
–Fragile bone
revise the terms exocrine, endocrine, paracrine and autocrine
Exocrine: Cells secrete substances via ducts which are then released onto epithelial surfaces
Endocrine: Cells producing hormones which are released directly into the bloodstream and circulatory system (so not via a duct) and act at distant sites.
Paracrine: When a signal induces changes in nearby cells, altering the behaviour of those cells
Autocrine: When a cell secretes a substance that binds to receptors on that same cell which leads to a change in the cell
identify these cells in the human pancreas
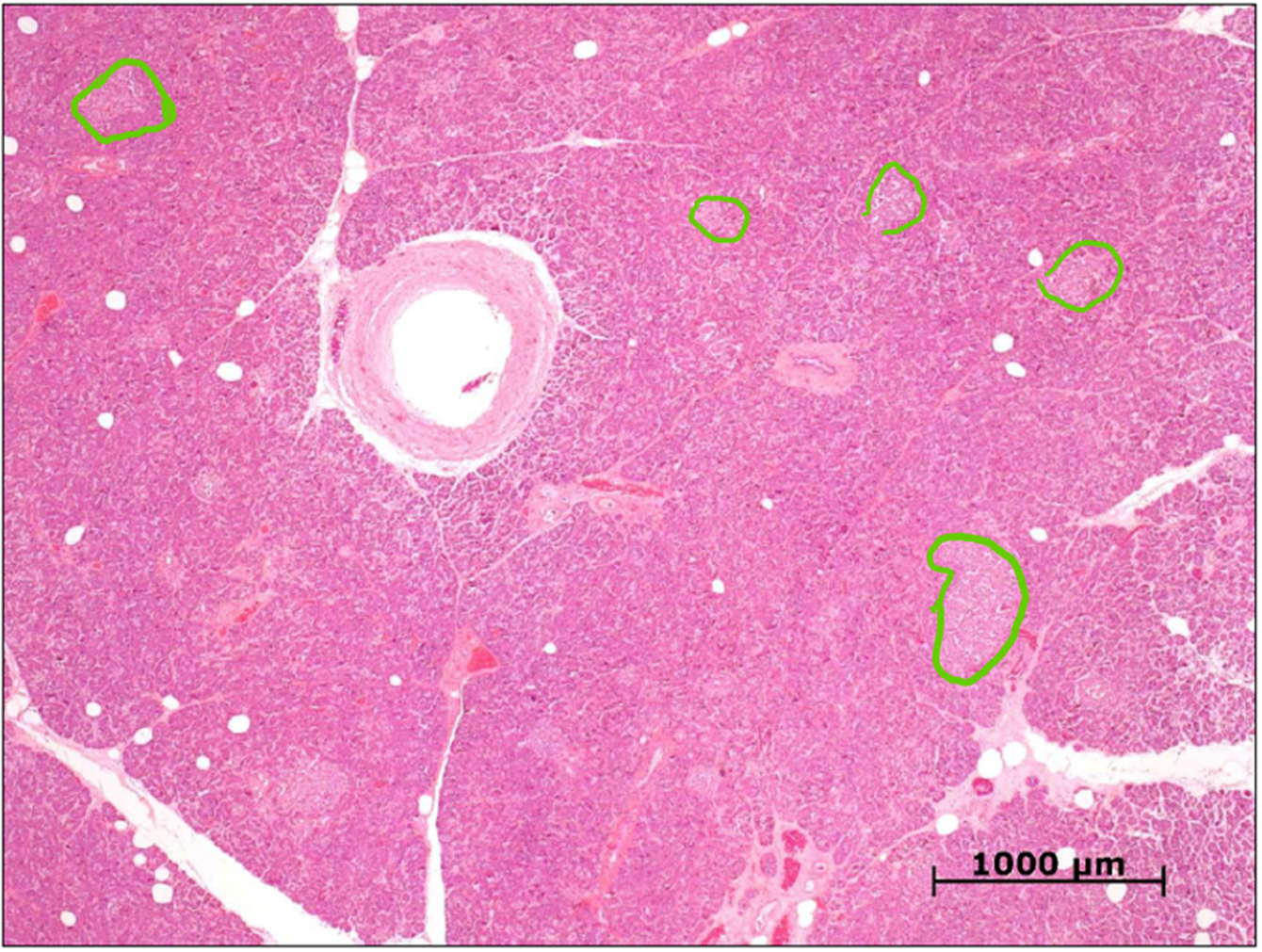
islets of Langerhans
(clusters of lighter stained areas)
endocrine
embedded in acini, separated by a fibro-collagenous capsule
most numerous in the tail region of the pancreas
describe the composition of islet of Langerhans
fibro-collagenous capsule separates the islets of Langerhans (endocrine cells) from the acini (exocrine)
this is an islet of Langerhans stained using Mallory's trichrome stain. Identify and differentiate between the blue stained cells and the pink stained ones
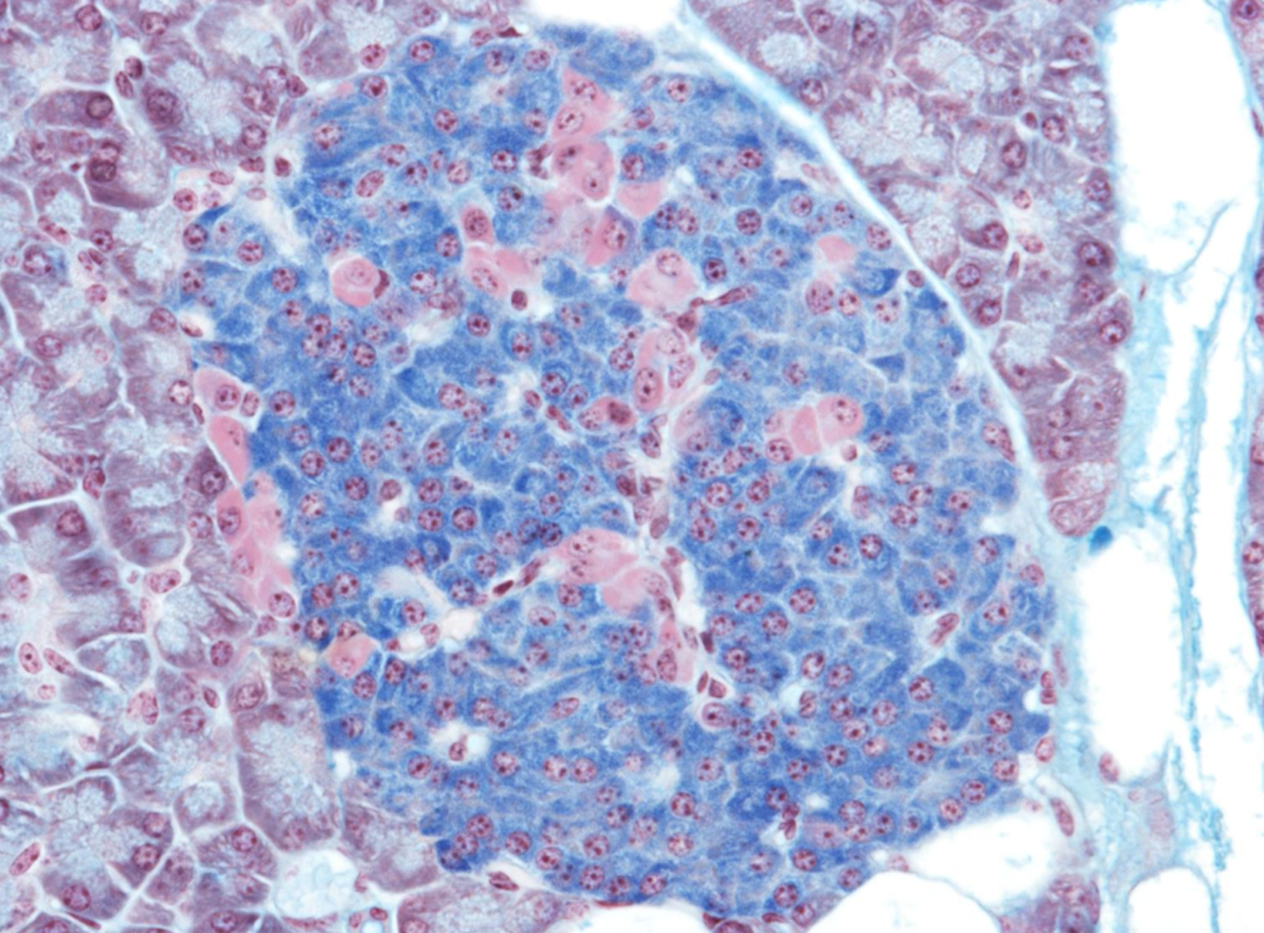
blue=Beta cells- insulin
pink=Alpha cells- glucagon
immunohistochemistry, antibodies specifically designed to identify antigens/proteins.
identify which one is insulin secreting cells (beta cells), and which one is glucagon secreting cells (alpha cells)

9a= beta cells- insulin
9b= alpha cells- glucagon
around 70% of the islet should be producing insulin
outline the ultrastructure of beta and alpha cells in islets of Langerhans
Rough Endoplasmic Reticulum (RER)
Beta cells= Well-developed, for protein synthesis (insulin)
Alpha cells= More extensive
Golgi Apparatus
Beta cells= prominent
Alpha cells= less prominent
neurosecretory vesicle- slightly smaller in alpha cells
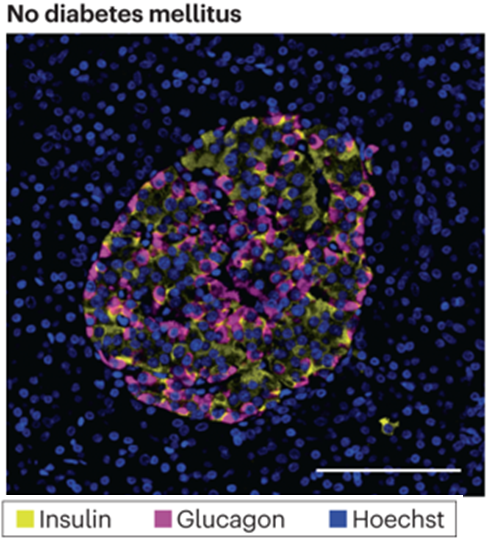
what would we see in microscopy Hoechst dye (nuclei=blue) in a patient with T1DM?
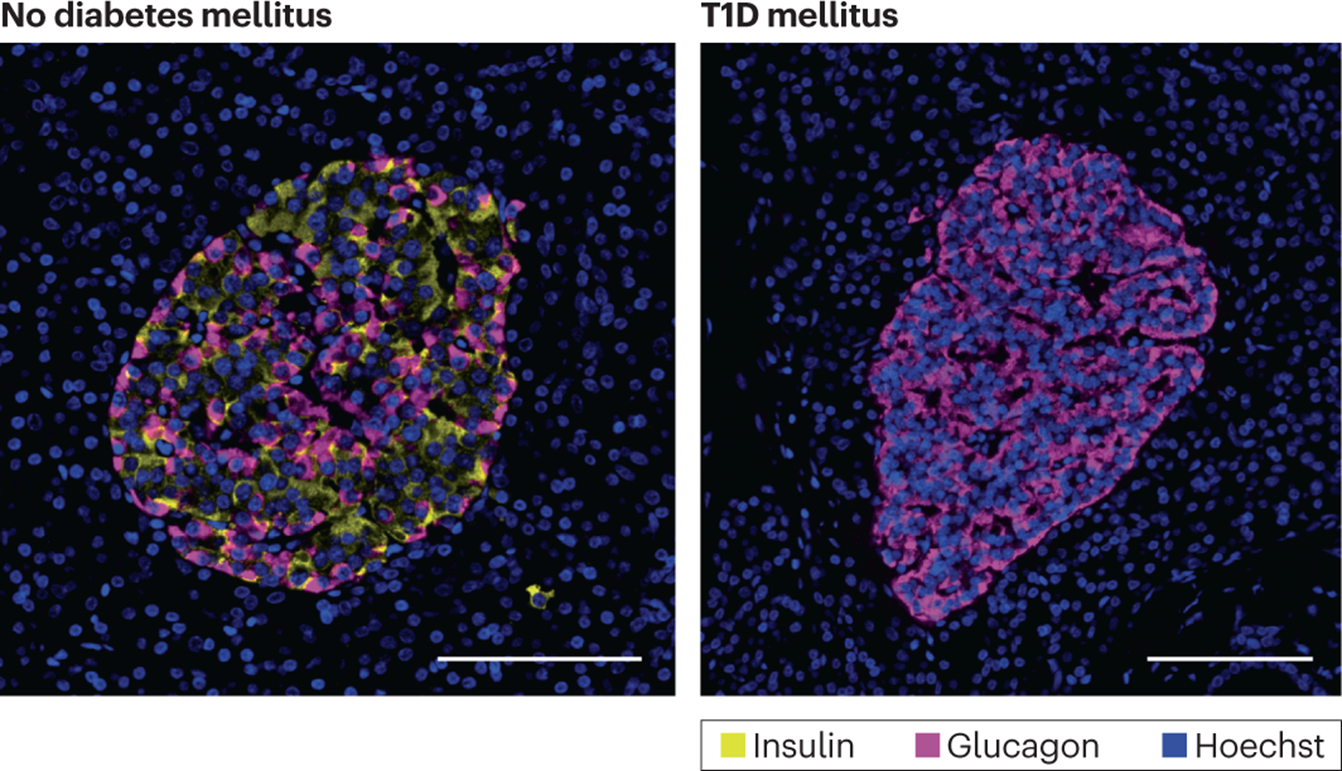
Which type of blood vessel passes through the red labelled area which is an Islet of Langerhans?
What can you say about the relative concentration of hormones within the blood entering the post-Islet exocrine pancreas?
Does the relatively high concentration of insulin influence activity of any other biomechanisms apart from uptake of glucose by cells of the body?
Which type of blood vessel passes through the red labelled area which is an Islet of Langerhans?
Small capillaries or venules
What can you say about the relative concentration of hormones within the blood entering the post-Islet exocrine pancreas?
The concentration of insulin and glucagon is high, proportions of each will vary with blood glucose content
Does the relatively high concentration of insulin influence activity of any other biomechanisms apart from uptake of glucose by cells of the body?
Yes, increased production of insulin by beta cells in the islets will stimulate / upregulate synthesis of exocrine pancreatic digestive enzymes for release into the duodenum via the pancreatic duct.
what condition of the pancreas does this image show?
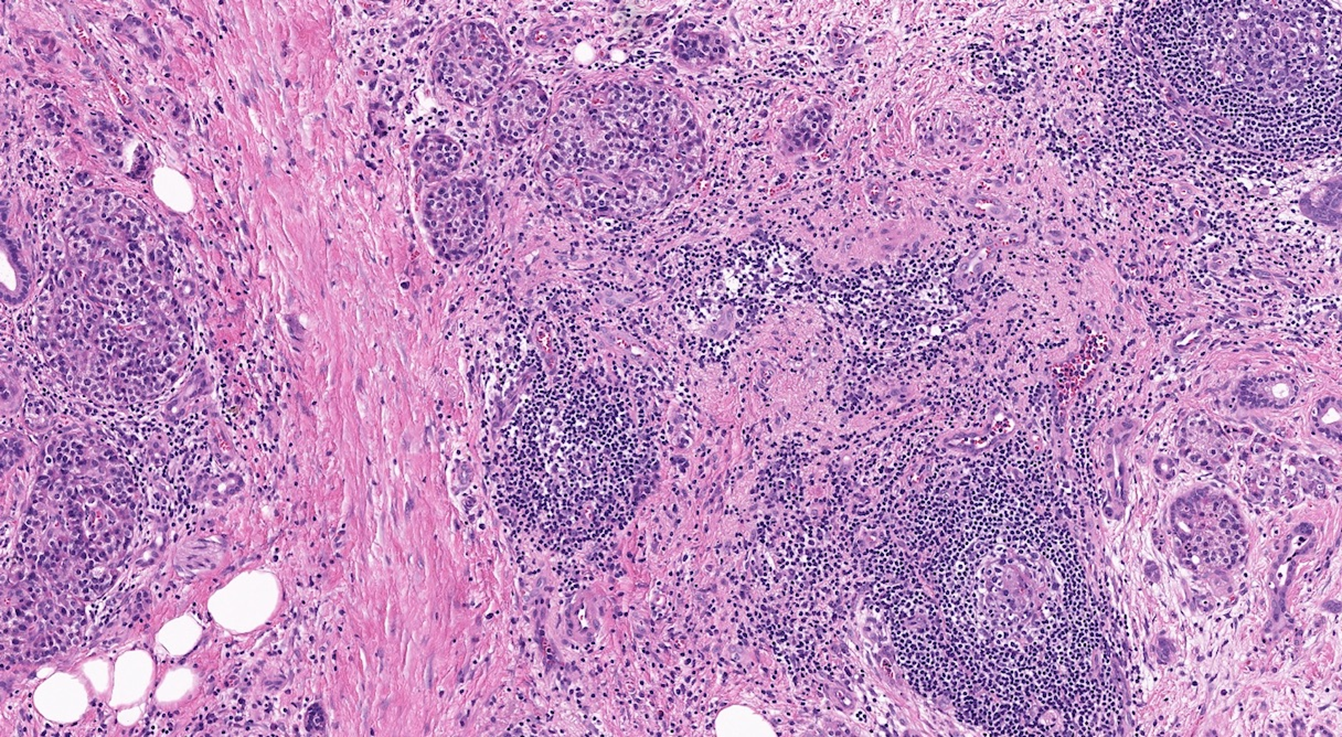
pancreatitis
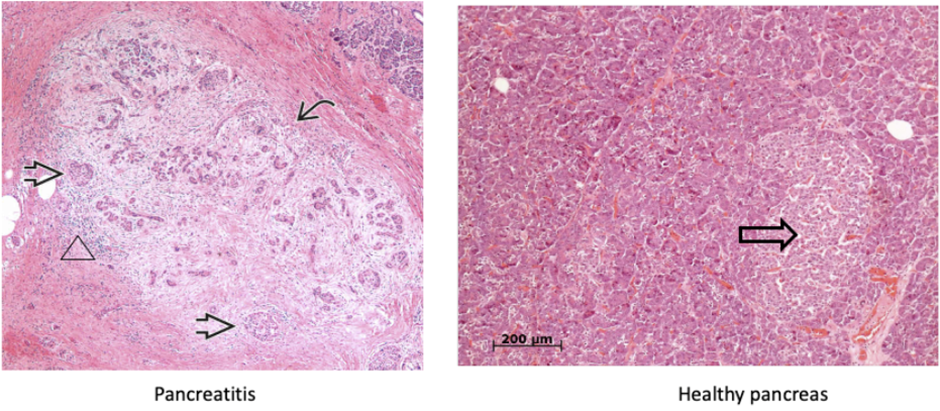
marked fibrosis and chronic Inflammation
marked loss of acinar parenchyma (curved arrow)
Residual islet cells and ductules are visible (arrows)
but eventually, these may become disappear and can lead to diabetes secondary to chronic pancreatitis.
the exocrine cells (acini) start breaking down first, endocrine cells (islet of Langerhans) break down on later stages
Loss of acini and ductal tissue with relative sparing of islets at early stages
Irregular areas of fibrosis which can obstruct the of pancreatic ducts
Chronic inflammation (including mast cells) around lobules and ducts (shown above by a triangle)
Dilated ducts with concretions
Ductal epithelium is atrophic, hyperplastic or undergoes squamous metaplasia
Islets may become sclerotic and disappear
If you wanted to stain fibrous material on histology slide, what stain would be good to use?
Masson's trichrome would be a good option, as it stains collagen a blue-green colour.
what is the diagnostic criteria for diabetes mellitus?
•Fasting blood glucose (FBG)
•< 6.0mmol/l: normal
•6.1-6.9mmol/l: impaired
•> 7mmol/l: diabetes
•75g Oral Glucose Tolerance test (OGTT) 2 hr glucose
•< 7.7mmol/l: normal
•7.8-11mmol/l: impaired
•> 11.1mmol/l : diabetes
•Need 2 abnormal tests, or 1+ symptoms
•HbA1c
•42-47mmol/mol: pre-diabetes/impaired glucose tolerance
•>48mmol/mol: diabetes
•Sample can be taken at anytime of the day-removes restrictions of fasting
•No glucose load required-quicker and less HCP time
-not for children, anyone with issues with their haemoglobins, anemia, on steroids
name all the types of diabetes
•Type 1 (prevalence 0.5%)
•Type 2 (prevalence 5%)
•MODY (autosomal dominant, prevalence 0.1%)
•Gestational Diabetes (GDM)
•Secondary Diabetes
•Pancreatitis (alcohol, gall stone, idiopathic, malignancy)
•Cystic fibrosis [affects secretions of the pancreas]
•Haemochromatosis
•Steroid-induced
•Acromegaly
what are the 2 main groups of diabetes, and examples of each?
•Insulin deficient
•Type 1
•MODY
•Pancreatitis
•Cystic fibrosis
•Haemochromatosis
•Insulin resistant
•Type 2
•Gestational
•Steroid-induced
•Acromegaly
which auto-antibodies cause T1DM?
•IA2 (insulinoma-associated antigen-2)
•GAD65 (glutamic acid decarboxylase 65)
•ZnT8 (zinc transporter)
•IAA (insulin auto-antibody)
•ICA (islet cell antibody)-less clinical utility
what is the diagnosis for T1DM?
•Initially: check Abs: GAD, IA2, ZnT8
•Abs-low titres can be seen in the gen population: can get ‘true false +ve’
•5% of PWT1DM are triple antibody negative
•Higher titres and multiple Ab +ve more discriminatory
•Later on (3y+):
•C-peptide >900 = T2
•C-peptide 200-900 = T1, T2 or MODY possible
•C-peptide-<200 = severe insulin deficiency
c-peptide is used to measure insulin production
what are some type 1 DM precipitating events?
•Enteroviruses (esp Coxsackie)
•Rotavirus
•Bacteria (eg Mycobacteria Avium paraTB)
•Environmental
•Cow’s milk
•Wheat proteins
•Vitamin D deficiency
•Insulin resistance (e.g. puberty)
•Psychological stress
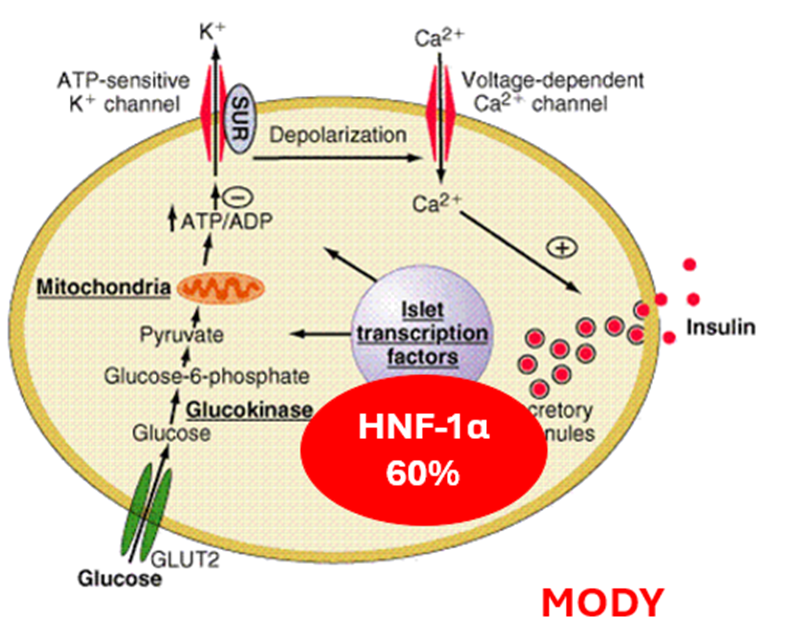
describe Monogenic Diabetes MODY (Maturity-onset Diabetes of the Young)
genes that decline insulin secretion within the cell.
HNF-1α
Glucokinase
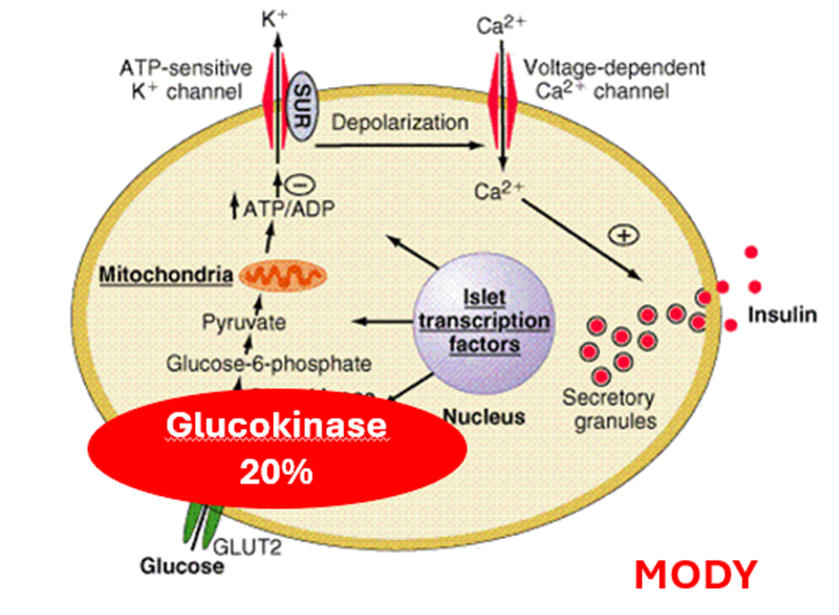
After 10y people with GCK mutation have no significant microvascular complications. GK mutation FBG 5.5-8mmol/L (Dx >7.0).
NO NEED FOR TREATMENT, if pregnant. the baby may have complications if they don’t have inherited the gene
HNF-4α
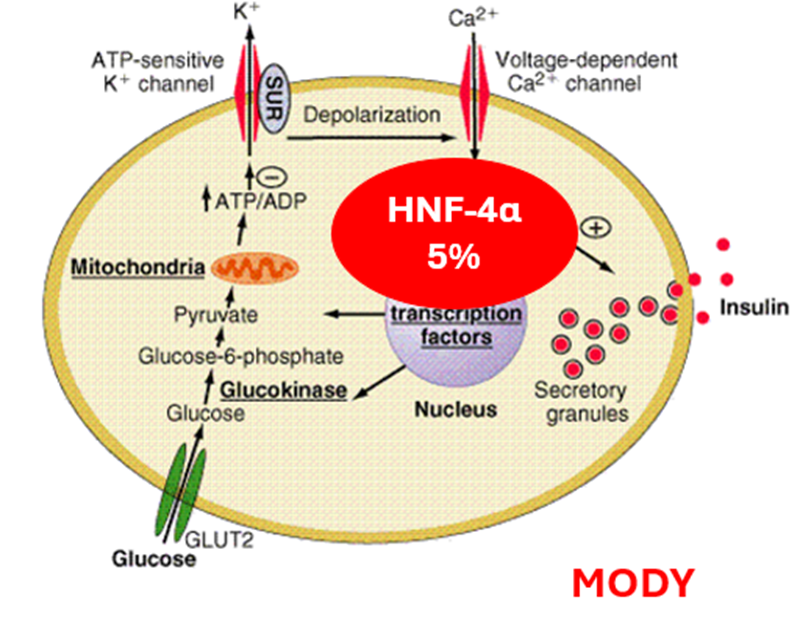
HNF-1β
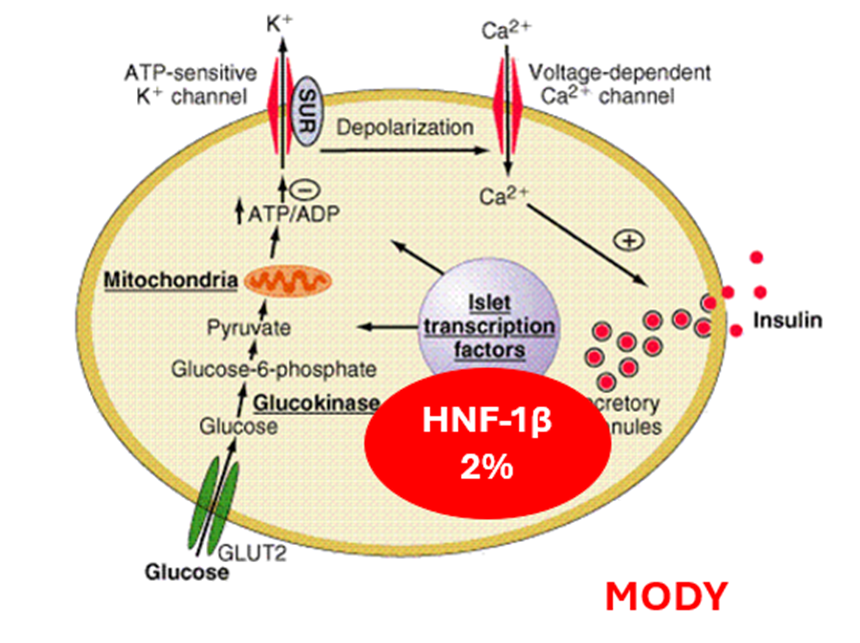
HNF1B protein found in many organs/tissues-lungs, liver, intestines, pancreas, kidneys, reproductive system, and urinary tract
HNF1B-plays a role in the development of many of these parts of the body.
Neonatal
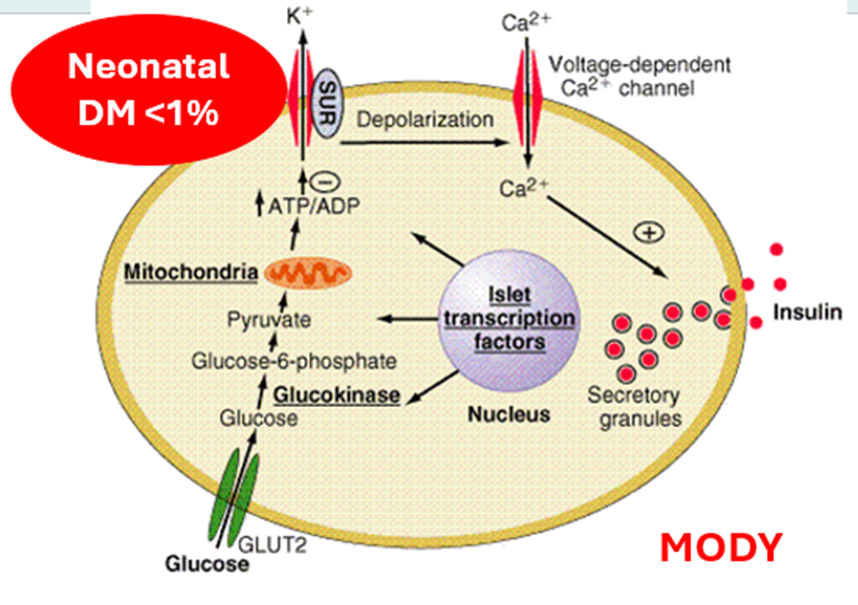
Most common genes: ABCC8 and KCNJ11 genes
~40% of patients with ND have a potassium channel gene mutation
Mutations in these two genes are sensitive to sulphonylurea treatment with better glycaemic control than insulin
which technologies are used for T1DM?
•How glucose is measured
Flash glucose monitoring (FGM)
Measures interstitial glucose
low and high glucose alarms
Continuous glucose monitoring (CGM)-initially ‘flash’
No swiping of sensor: SG every 5min
Interstitial fluid glucose
Data sent to pump, phone, smart watch
Ambulatory Glucose Profile (AGP)
•Remote data review
•New metrics: Time in range (TIR), Glucose variability
•How insulin is delivered
•Insulin pumps/CSII (continuous subcutaneous insulin infusion)
Closing the loop
•Hybrid close loop (HCL) or automated insulin device (AID)
connects: insulin pump to CGM (wirelessly), using a computer algorithm
automatically adjusts insulin delivery based on SG readings
still requires user interaction for meals and exercise