Metal Alloys for Removable Partial Dentures (RPDs) and Clasps
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
For the last lecture
Dentures rely on soft tissues while fixed pros don’t so it doesn’t matter if you use a viscous muco/compressive impression material
immediate casting for alginates
Elastomers vary on whether they are compressive or static based on high viscosity and low viscosity respectively
might use putty instead of green stick for border moulding
Polyether - hydrophilic
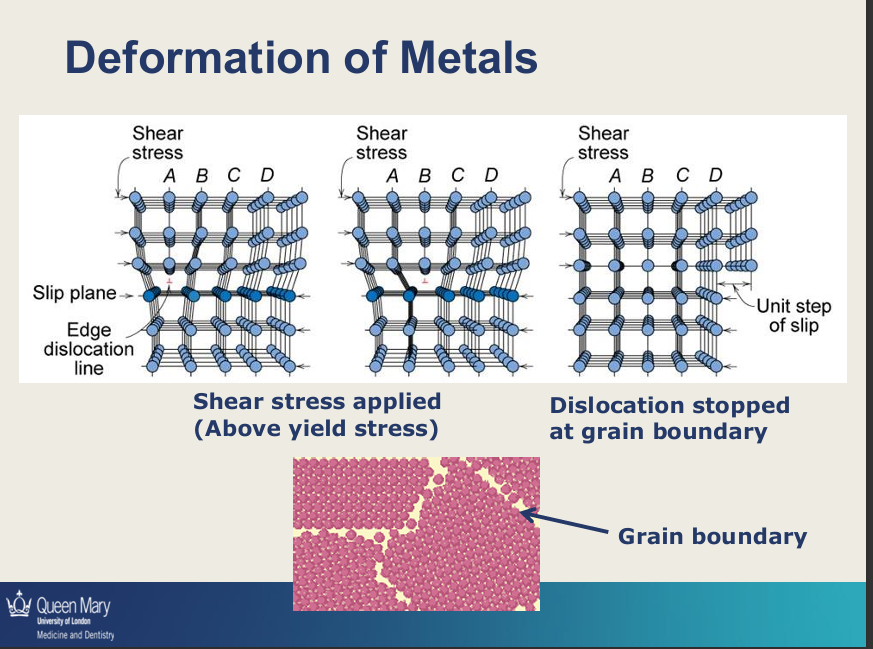
Deformation of metals requires what?
what happens in the metal in permanent deformations?
to deform metals you apply stress above yield stress
if it is lower than the yield stress, you get no deformation
you get movements of dislocations which then stop at the grain boundary
in the image there is an extra plane of atoms moving from A to B to D and keeps moving due to application of stress above the yield stress - as it moves it will eventually stop at the grain boundary - as between grain boundaries structure is amorphous where atoms are arranged randomly so cannot move beyond the crystalline structures (where atoms are arranged in organised structure).
next time you deform the metal you require higher forces
when you bend/apply force the metal more dislocations are also made
eventually it will fracture
What is work (strain hardening)?
Application of shear stresses produces more dislocations, hinders the movement of dislocations, more difficult to deform the metal
What are other strengthening mechanisms? (6)
Forging - drawing wire/rolling sheets
Bending - denture clasp, orthodontic appliances
burnishing - amalgam or Gold Inlay
Solution hardening - different atomic radii of atoms within same crystalline structure limit the movement of slip planes (alloys)
order hardening - super lattice (ordered structure) formation (in a solid solution)
precipitation hardening - partial solubility varying with temperature Ag-Cu
in a solid solution the metals are completely soluble
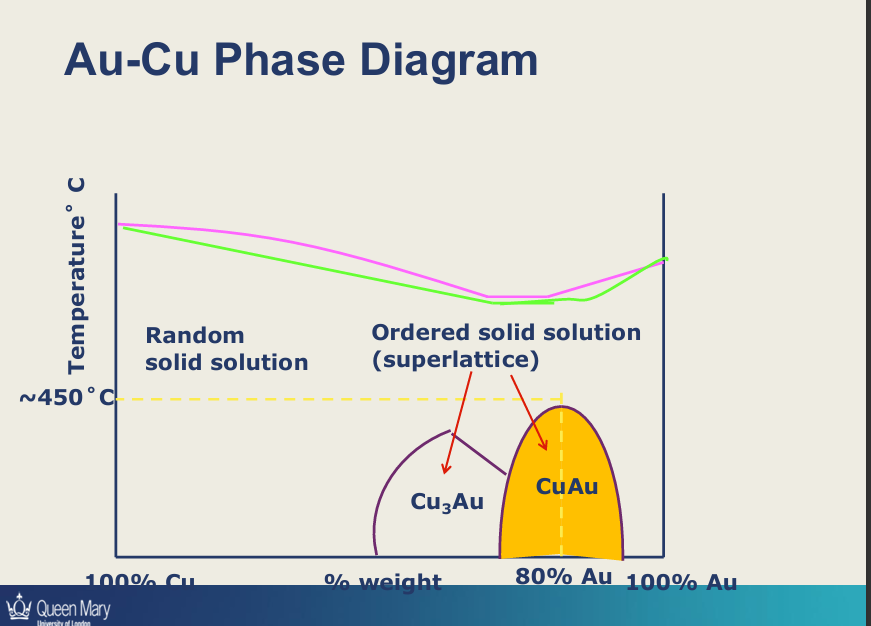
Describe this diagram
heat above the pink line - liquid
fast cooling/quench/solid solution - 1 phase of metal in a random state (organised/ordered/crystalline structure but atoms in random structure) (atom position is disordered)
put the metal under heat treatment - not melting the metal - giving atoms energy and time to form ordered structure within the crystal = super lattice (atoms occupy specific repeating positions)
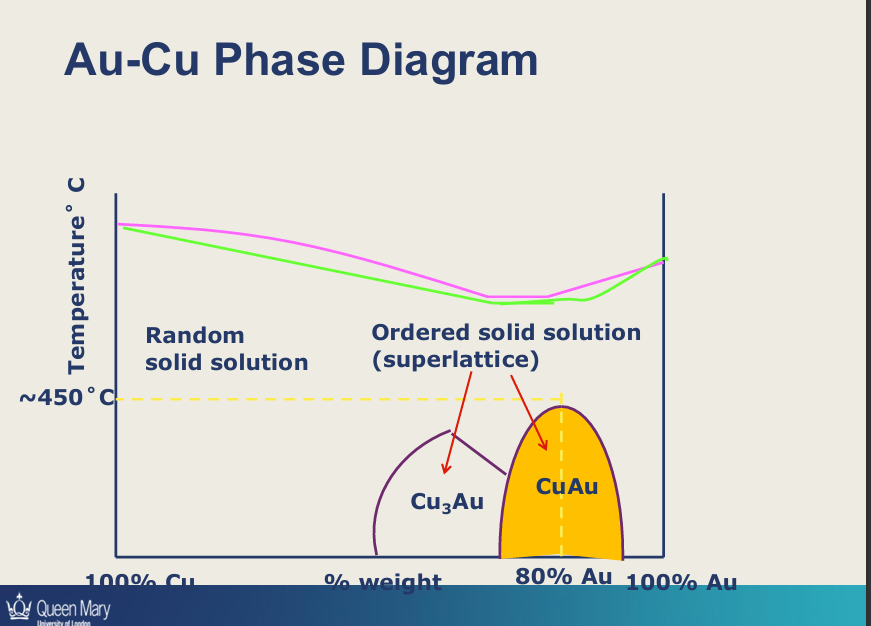
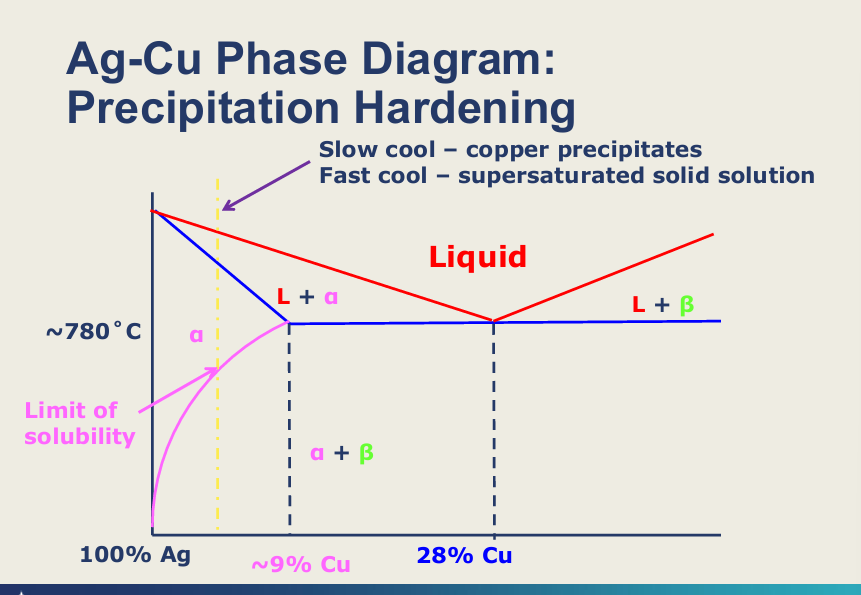
Explain this diagram?
Solid solution - 2 metals are completely soluble in each other so one phase
limit of solubility, if you cool metal you freeze structure in place (alpha) - supersaturated solution
if you slow cool and below limit of solubility - you get a phase rich in copper will start precipitating within the alloy - so you create obstacles that dislocations can’t move beyond that second phase
slow cooling allows the Cu to diffuse when the solubility decreases

What are the 3 classifications of dental alloys? (3)
High noble
Noble
predominantly base metal
What is a Noble Metal?
Must contain a Noble metal
Containing least 60% noble metal of which 40% must be gold Au
Other metals - Pt, Pd, Rh, Ru, Ir, Os
platinum, palladium, rhodium, ruthenium, iridium, osmium

Noble metals?
Must contain at least 25% noble metals
Predominantly base metals?
Contain <25% noble metal
What are the alloys used for removable partial dentures?
Initially
1930’s
1970’s
other alloys (3)
Gold alloys
chrome-based developed
cobalt-chrome alloys
nickel chrome, type IV gold, titanium-based alloys

3 main categories for ideal requirements of Removable partial dentures alloys?
Handling and fabrication
Mechanical properties
Biological and clinical
Handling and fabrication requirements includes: (7)
Easy to cast (low melting point, high density)
High density = easier to fill mould completely and force air out of the mould
Easily soldered or laser-welded
Low shrinkage
Easy to finish, polish and adjust
Mechanical properties requirements includes: (4)
High rigidity (high modulus) - support and stability
High yield stress and fatigue stress - avoids permanent deformations during function
Good wear resistance
Biological and clinical ideal requirements include? (4)
Biocompatible and corrosion resistant
Comfortable (light weight thin sections)
Aesthetic compatibility with acrylics and tissues
What type is high gold alloy? 1-4?
Type 4 (IV)
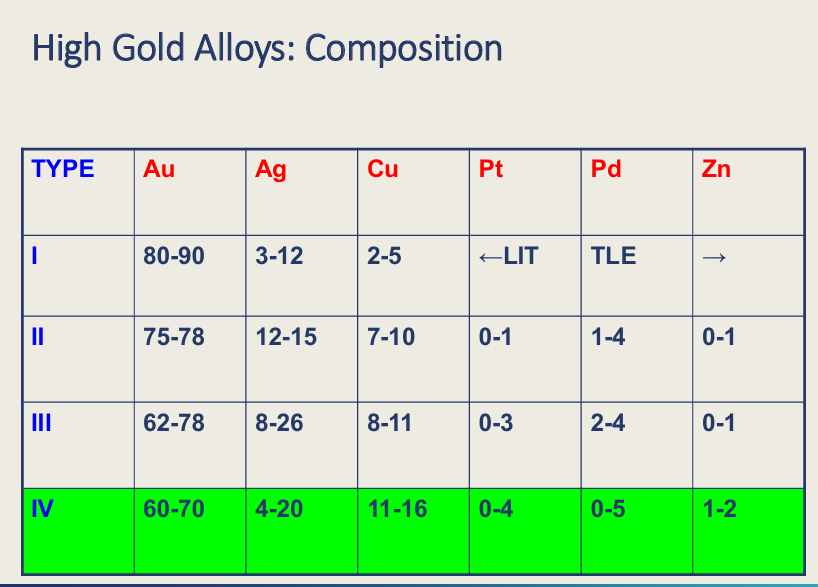
3 and 4 further divided into?
cast and hard

Which have the better property?
hard is more hard and higher tensile strength the 4 has better properties Don’t have hard type 1 and type 2 - hard - not enough copper in the system for ordered structure
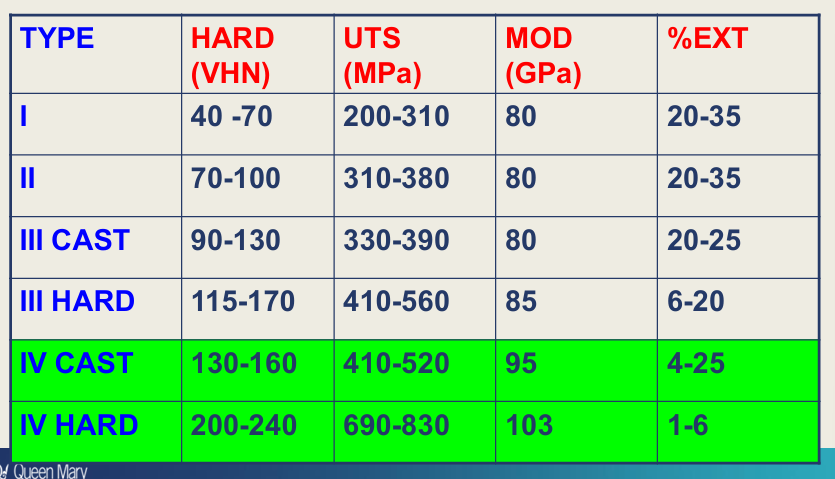
Properties of type IV gold for removable partial dentures (not used anymore): adv(7) dis(4)
Adv:
Low melting point
Easy to cast
easy to solder
can be heat hardened (order hardened)
easy to finish/polish
biocompatible
corrosion resistant
dis:
High density
Low yield stress
low modulus
expensive
high density and low modulus - needs to be in thick cross sections - not comfortable for patient
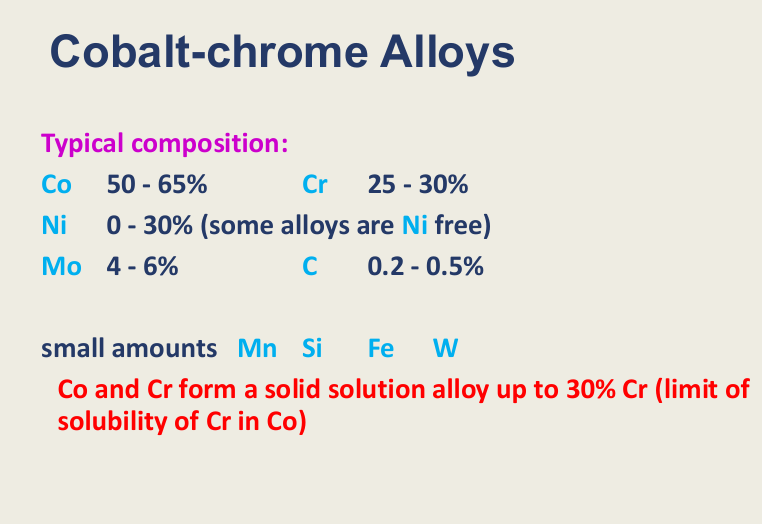
Typical composition of Co-Cr alloys
what is the maximum amount of Chrome?
other metals that can be involved? (3 main and 4 others)
Co - 50-65%, Cr - 25-30%
Ni 0-30% (some without) (replaces some of the Cr)
Mo 4-6% or C 0.2-0.5%
Small amounts of: Mn, Si,Fe,W
Co and Cr forma a solid solution alloy up to 30% Cr - limit of solubility of Cr in Co
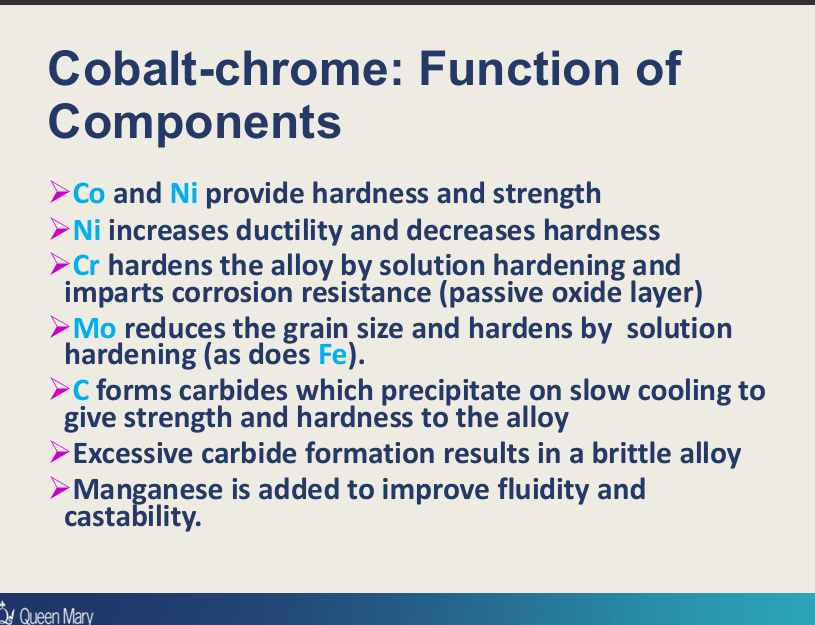
What are the functions of Co, Ni, Cr, Mo, Fe, C, Mn in the Co-Cr metal framework?
Cobalt:
hardness and strength
Ni:
hardness and strength (in Ni-Cr)
increases ductility and decreases hardness (C to Co in Ni-Cr alloys)?
Cr:
hardens the alloy by solution hardening,
corrosion resistance by passive oxide layer
Mo+Fe:
reduce grain sizes
hardens by solution hardening
C:
forms carbides which precipitates on slow cooling to give strength and hardness (excesses results in brittle alloy - therefore added in small amounts)
Mn:
improve fluidity and castability
Adv of Co-Cr? (7)
Low cost compared to gold alloys
hard
abrasion resistant
High modulus - can be used in thinner sections
low density - light weight
high yield stress - less likely to become permanently deformed
Ni free are biocompatible
Dis of Co-Cr ? (5)
High casting temperature
casting shrinkage - 2%
Limited ductility - clasps may fracture if adjusted
rapid work hardening - limited chairside adjustment
Ni sensitivity
difficult to finish/polish
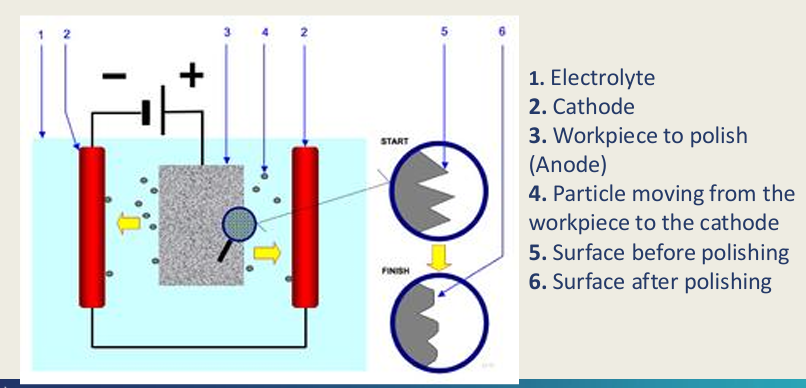
Electroyle polishing of fit surface?
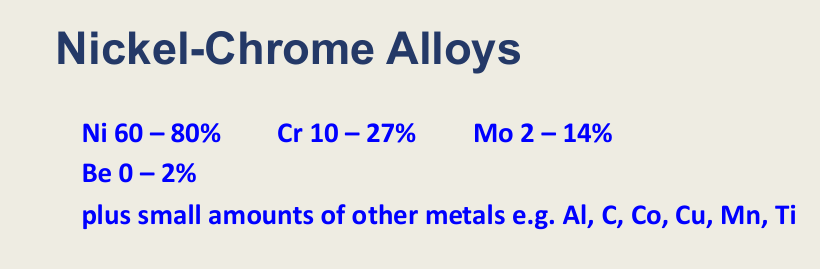
Ni-Cr alloys composition (4 main) small amounts of other metals? (6)
Ni, Cr, Mo, Be
Al, C, Co, Cu, Mn, Ti
The purpose of Ni?
Cr?
what to note about C addition?
Ni:
Strength and hardness
Cr:
Hardens solution by solution hardening
Corrosion resistance - passive oxide layer
must also limit carbide precipitation for strength
Properties of Ni-Cr alloys similar to those of?
CO-Cr
Ni-Cr has largely been replaced by Co-Cr and Ti alloys
Titanium and alloys 2 types?
Commercially pure and alloys

What can titanium and alloys be used for? (4)
Dental implants
Crown
Bridges
Partial dentures
Generally:
High density - low MP
Difficult to cast - high MP - low density - high shrinkage
Titanium and alloys properties? (8)
Biocompatible
Low density
Excellent corrosion resistance - passive O layer
High melting point
Well defined fatigue limit - highest for alloys
difficult to cast
high casting shrinkage - 3.5%
can react with investment material

How can commercially pure titanium be classified, what elements might be included (5) and % of titanium in CpTi?
4 grades containing +99% Ti, N, C, H, Fe, and O
Grade 1 - 4
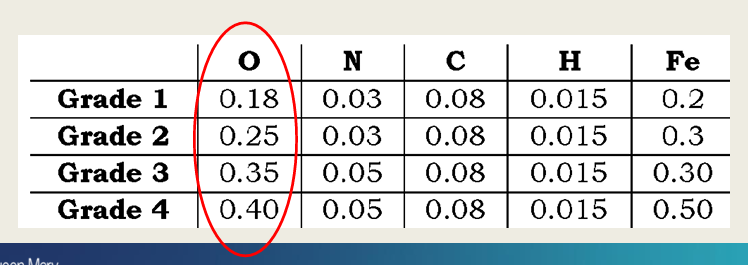
How does increasing O affect the titanium material?
increases X and decreases Y?
Increasing O increases strength and decreases ductility
Titanium alloys:
alloyed with what metals?
Al or Nb (niobium) or V (vanadium)
2 forms of Ti?
alpha - low temp
Beta - high temp form
What is the form found in Cp titanium? what elements are need to stabilise this form?
Cp titanium alpha form is stabilised by O, C,N
What forms are found in Ti6AL4V alloys and what stabilises it?
Alpha and beta - V stabilises the beta form, Nb in TiAL7Nb will stabilise the beta
What is the problem with V?
vanadium is toxic in elemental form, can be replaced by Nb - Ti6Al7Nb
Titanium framework are now made by what methods ? (2)
Digital dentistry:
Computer numerical control (CNC) milling
Laser sintering SLM for RPDs
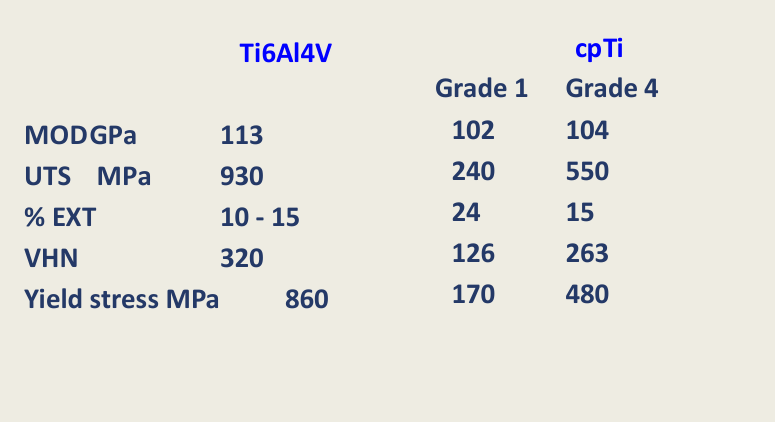
Properties similar to ?
CpTi
Which has better properties, the titanium alloy or the commercially pure titanium
Improved properties in titanium alloys comparedto CpTi
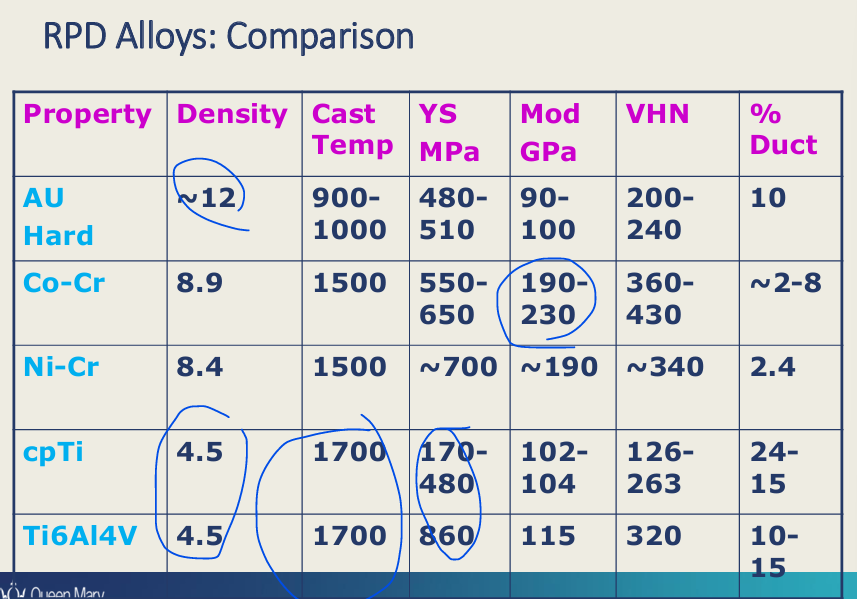
comparison of alloys used for RPDs
2 methods of making clasps?
Wrought clasps attached by soldering
Casting with framework
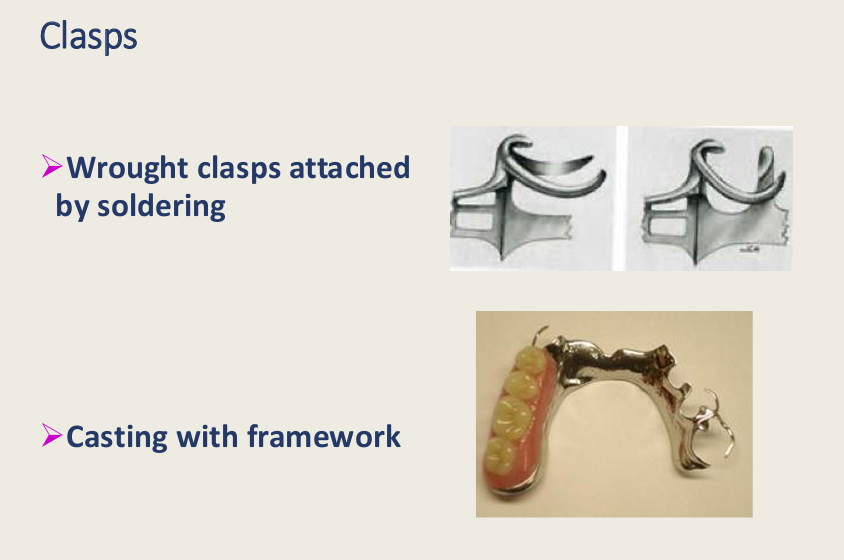
Which materials are used for cast clasps and wrought clasps (attached by soldering?), properties of each, 1 adv and 1 dis for each?
Rapid work hardening in Co-Cr - hence fracture if bent
Easily adjusted so better fatigue
flexible - better for undercuts

Aesthetic and tooth-coloured clasps - use which materials? (3)
Acetal resin (Polyoxymethylene)
Polyamide (Nylon)
PEEK/PEKK (polyetheretherketone polymers)
What are the adv of aesthetic and tooth-coloured clasps?
tooth coloured - improved aesthetic
Flexible - engage deeper undercuts
Biocompatible - metal-free
can be used with metal or acrylic frameworks
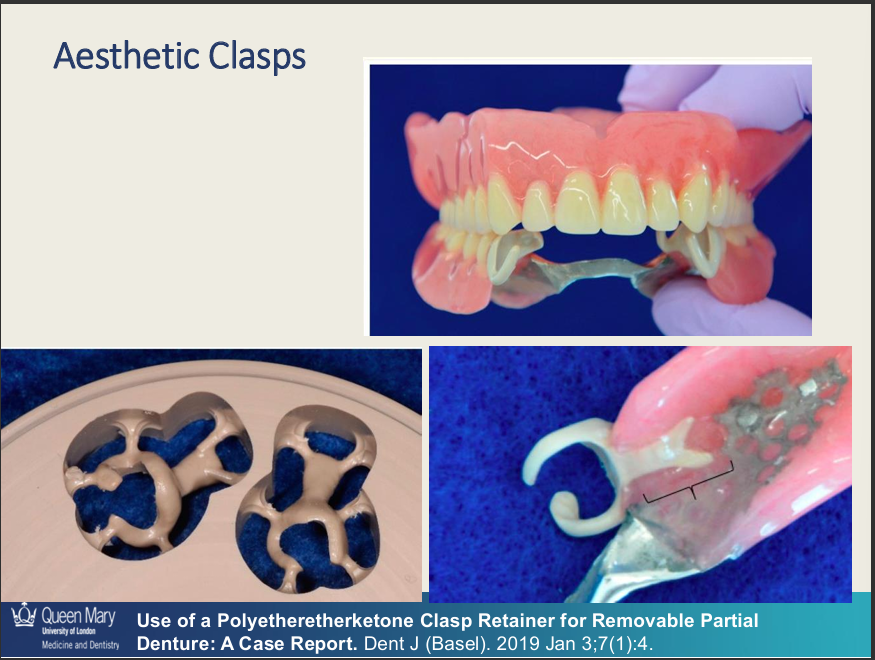
3 dis of aesthetic and tooth-coloured clasps?
lower rigidity - limited support
may discolour over time
harder to polish than metal
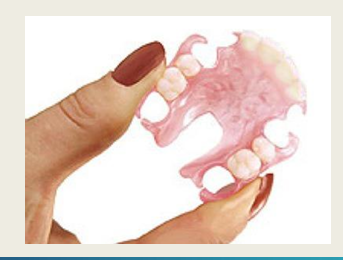
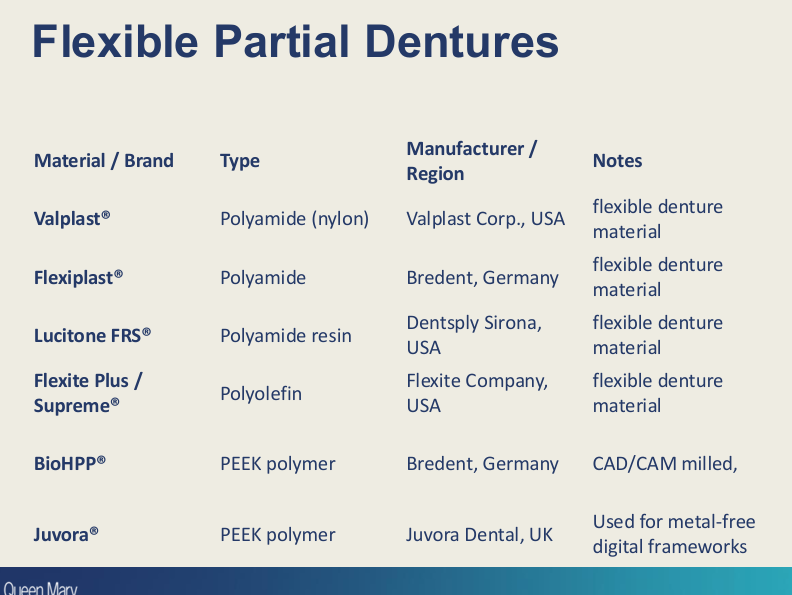
Flexible partial dentures:
what materials are commonly used?
Polyamide (Nylon) - e.g valplast
Acetal resin - polyoxymethelene
Peek/pekk - polyetheretherketone

Fabrication method? (2)
Injection moulding - traditional
CAD/CAM milling or 3D printing (modern)
Adv of flexible partial dentures? (4)
Flexible - engage undercuts without stress
lightweight and comfortable
aesthetic - no visible metal
biocompatible - no residual monomer
Dis of flexible partial dentures? (3)
usually used as what?
Not as rigid - limited support and stability
Prone to staining or warping overtime
Difficult to repair or reline
Usually used as interim or provisional solution
What is soldering How does it work?
The joining of metals by a filler metal (solder)
solder melts and wets the surface and joins 2 metals together
What must be used first before the solder?
Flux, as it dissolved the surface oxides
it wets the surface
and solder displaces the flux
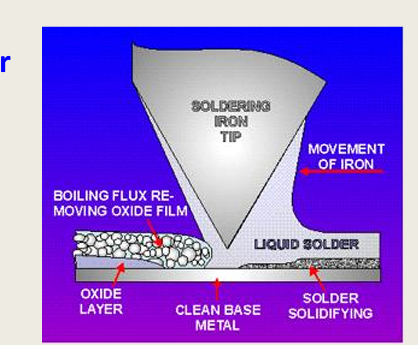
what happens to the metal once passive oxide layer is removed?
metals have high surface energies
attract the molten solder to form bonds
Fluxes are commonly what?
give examples used with gold alloys (4)
and one example used with base metals alloys (1)
Borates
gold alloys: borax/sodium tetraborate/borax glass, sodium pyroborate
fluoride fluxes are used with base metal alloys
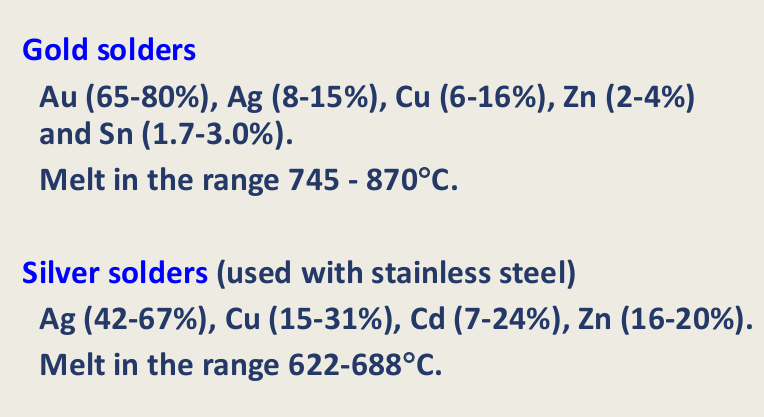
Hard dental solders need to be (4)
Corrosion resistant
Have a high fusion temp - 50 degrees less than alloy (Don’t want it to change shape/melt)
be as strong as the alloy
have good flow
2 types of Solder
what is one of them typically used with?
which has the higher melting range?
Gold solders
Silver solders - stainless steel
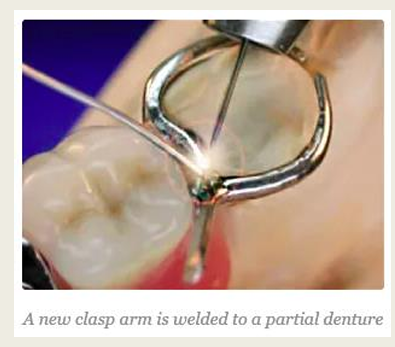
What is welding?
Joining metals without the use of a solder
3 types of welding?
Pressure welding - metals are heated to recrystallisation temperature under pressure
Electric spot welding - electric current generates heat at contact points
laser welding (modern technique)- uses a focused laser beam to melt and fuse metals precisely
Pressure welding is used for and not used for?
used for orthodontic wires not RPD frameworks
Electric spot welding is limited to?
thin metal sheets or wires
Laser welding is suitable for which materials used for RPD, 2 adv?
gold, Co-Cr, SS, Ti
minimal heat distortion and high accuracy
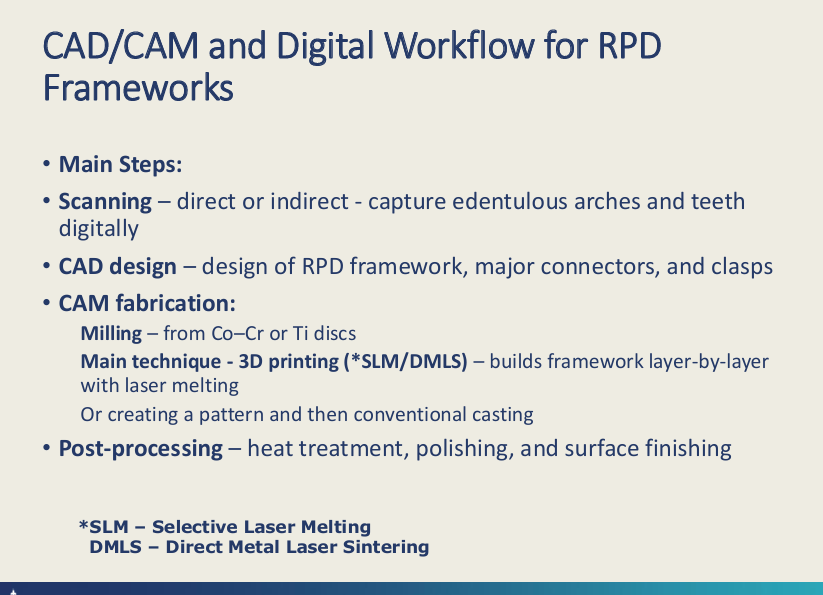
CAD/CAM workflow for RPD frameworks: main steps? (4 main)
Scanning
CAD
CAM:
- milling
-SLM/DMLS selective laser melting or direct melting laser sintering
creating pattern then conv casting
Post-processing - heat tx, polishing and surface finish
ADV of CAD/CAM? (3)
short time production
digital storage and easy remakes
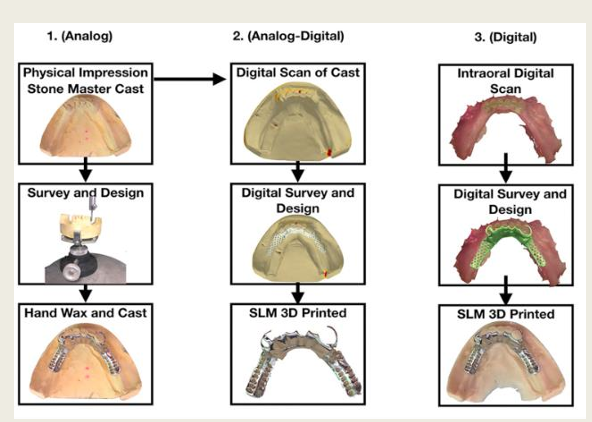
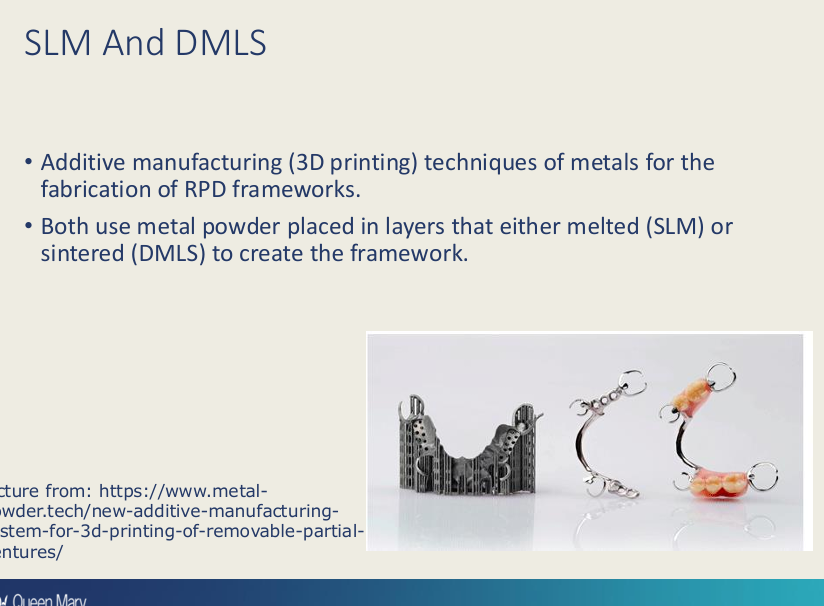
Difference between selective laser melting and direct metal laser sintering?
what type of manufacturing technique are they?
both use what?
main difference
additive
metal powder in layers
In SLM - melted
In DMLS - sintered