AP Biology Summer Homework
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
What are the seven properties associated with life?
order
regulation/homeostatsis
growth and development
energy processing
response to the environment
reproduction
evolution/adaptation
Differences between prokaryotic and eukaryotic cells
Prokaryotic cells are smaller, have DNA in their cytoplasm, and reproduce asexually
Eukaryotic cells are bigger, have DNA in their nucleus, and reproduce sexually & asexually
What is the "Form fits Function" concept?
the shape and structure of a biological entity is related to its specific role or job in its environment or organism. For example, the shape of a bird's beak is adapted to its feeding habits.
What are the five kingdoms of life?
Bacteria (single-celled prokaryotes)
Protista (single-celled eukaryotes)
Fungi (multicellular eukaryotes that absorbs food from orgnic materials)
Plantae (multicellular photosynthetic organisms)
Animalia (multicellular organisms that ingest food).)
What is the scientific method?
1) observe
2) forming an hypotheses
3) experiment
4) analyze data
5) drawing conclusions
What were the controlled variables in the snake mimicry experiment?
factors such as the background color and size of the snake models were held constant while varying the color patterns to observe predator responses.
What four elements make up 96% of living matter?
carbon, hydrogen, oxygen, nitrogen
How many electrons does carbon have and why is this important?
Carbon has 6 electrons. This is important because this allows carbon atoms can form up to four covalent bonds with other atoms, allowing for the formation of complex organic molecules, which are the basis of biological macromolecules like proteins, carbohydrates, lipids, and nucleic acids.
What is a covalent bond?
A chemical bond formed when two or more atoms share electrons.
What is an ionic bond?
A chemical bond that's formed when an atom transfers an electron to another atom
What is an atom's mass number?
sum of protons and neutrons in the nucleus
What is an atom's valence?
the number of covalent bonds it can form
What is the only subatomic particle involved in chemical reactions?
only electrons participate in the formation and breaking of chemical bonds
Why are radioisotopes important to biologists?
used as tracers in biological research to track molecules and processes, as well as in medical imaging and cancer treatments
What is the octet rule?
atoms tend to gain, lose, or share electrons until they have a full outer shell of eight electrons (or two in the case of hydrogen), achieving stability
Why are noble gases unreactive?
Their outer energy level of electrons is full. They do not react with any other elements because they don't need any electrons and don't want to give any away either.
What are the different types of chemical bonds?
Nonpolar covalent bonds: Electrons are shared equally between atoms.
Polar covalent bonds: Electrons are shared unequally, creating partial charges on atoms.
Ionic bonds: Electrons are transferred from one atom to another, resulting in ions held together
How do hydrogen bonds form, and how are they different from covalent and ionic bonds?
Hydrogen bonds form when a hydrogen atom, which is bonded to a highly electronegative atom, is attracted to another electronegative atom. This creates a weak bond between molecules, making these bonds different from covalent or ionic bonds.
Sketch a water molecule, showing oxygen's electron shells and the covalently shared electrons. Indicate the areas with slight negative and positive charges that enable a water molecule to form hydrogen bonds with other polar molecules.
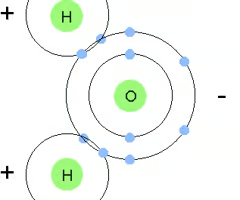
Why are weak bonds important to living organisms?
Weak bonds can be easily broken a reformed. When many weak interactions occur together they can be powerful. The weak bonds together can reinforce the 3D shape of the molecule, enabling them to carry out specific functions in biological systems.
What is the order of bonds from strongest to weakest?
Covalent bonds > Ionic bonds > Hydrogen bonds > van der Waals interactions
What is the balanced equation for respiration?
C6H12O6 + 6O2 → 6CO2 + 6H2O
How were the chemical conditions of early Earth different from today?
Early Earth had a reducing atmosphere (lacked free oxygen), high levels of volcanic activity, and frequent lightning, creating an environment rich in water vapor, hydrogen gas, methane, and ammonia. This is very different from today's oxidizing atmosphere with abundant oxygen and stable nitrogen levels.
What is an ion?
A charged atom
What is a cation?
A positively charged ion
What is an anion?
A negatively charged ion
What is an isotope?
Atoms of the same element that have different numbers of neutrons
What does half-life mean?
It is the amount of time for half of a given radioactive source to decay.
How does water contribute to the fitness of the environment to support life?
It acts as a solvent, participates in chemical reactions, regulates temperature, supports organisms through buoyancy, and facilitates transport of nutrients and waste.
Describe the geometry of a water molecule.
The molecule has a bent shape (angular geometry) due to the two lone pairs of electrons on the oxygen atom.
What five properties of water result from hydrogen bonding.
Cohesion and surface tension
High specific heat
High heat of vaporization
Expansion upon freezing (ice floats)
Versatility as a solvent
What is the difference between cohesion and adhesion?
Cohesion is the attraction between molecules of the same substance (like water molecules sticking together), while adhesion is the attraction between molecules of different substances (like water molecules sticking to other surfaces).
What is the biological significance of the cohesiveness of water?
Cohesion allows water molecules to move upward against gravity in plants (capillary action), ensuring efficient transport of water and nutrients from roots to leaves.
What is the benefit of water's high specific heat?
It moderates temperature changes in aquatic and terrestrial environments, stabilizing climate and supporting life. High heat of vaporization moderates temperature changes during evaporation, cooling organisms and environments.
What's the difference between a solvent and solute.
A solvent is the solution that dissolves the solute.
How does the polarity of water make it a versatile solvent?
Allows it to dissolve a wide range of substances, particularly those with ionic or polar covalent bonds, making it essential for biological processes like nutrient transport, metabolism, and waste removal.
Indicate whether the following are hydrophilic or hydrophobic. Do these substances contain ionic, polar covalent bonds or nonpolar covalent bonds?
olive oil
sugar
salt
candle wax
Olive oil: hydrophobic (contains nonpolar covalent bonds)
Sugar: hydrophilic (contains polar covalent bonds)
Salt: hydrophilic (contains ionic bonds)
Candle wax: hydrophobic (contains nonpolar covalent bonds)
How do acids and bases directly or indirectly affect the hydrogen ion concentration of a solution?
Acids: Increase H+ ions by adding them directly to the solution.
Bases: Decrease H+ ions either by accepting them or by releasing OH- ions, which then combine with H+ ions to form water.
How do buffers work? Use the bicarbonate buffer system as an example.
A buffer system is a mixture of a weak acid and its corresponding base that helps maintain a stable pH in a solution. It works by neutralizing added acids or bases, preventing drastic changes in pH. For example, the bicarbonate buffer system in blood helps keep pH levels steady, which is crucial for proper bodily function.
What is inductive and deductive reasoning?
Inductive reasoning uses data to draw conclusions while deductive uses theories
What is a polymer?
a large molecule that is made by combining smaller units called monomers.
What is a macromolecule?
biologically important molecules that are typically formed by polymerization (a polymer is a large molecule that is made by combining smaller units called monomers, which are simpler than macromolecules)