Topic 13- Alkenes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
How does a pi bond form?
The p orbitals overlap.
What is the Electrophilic mechanism?
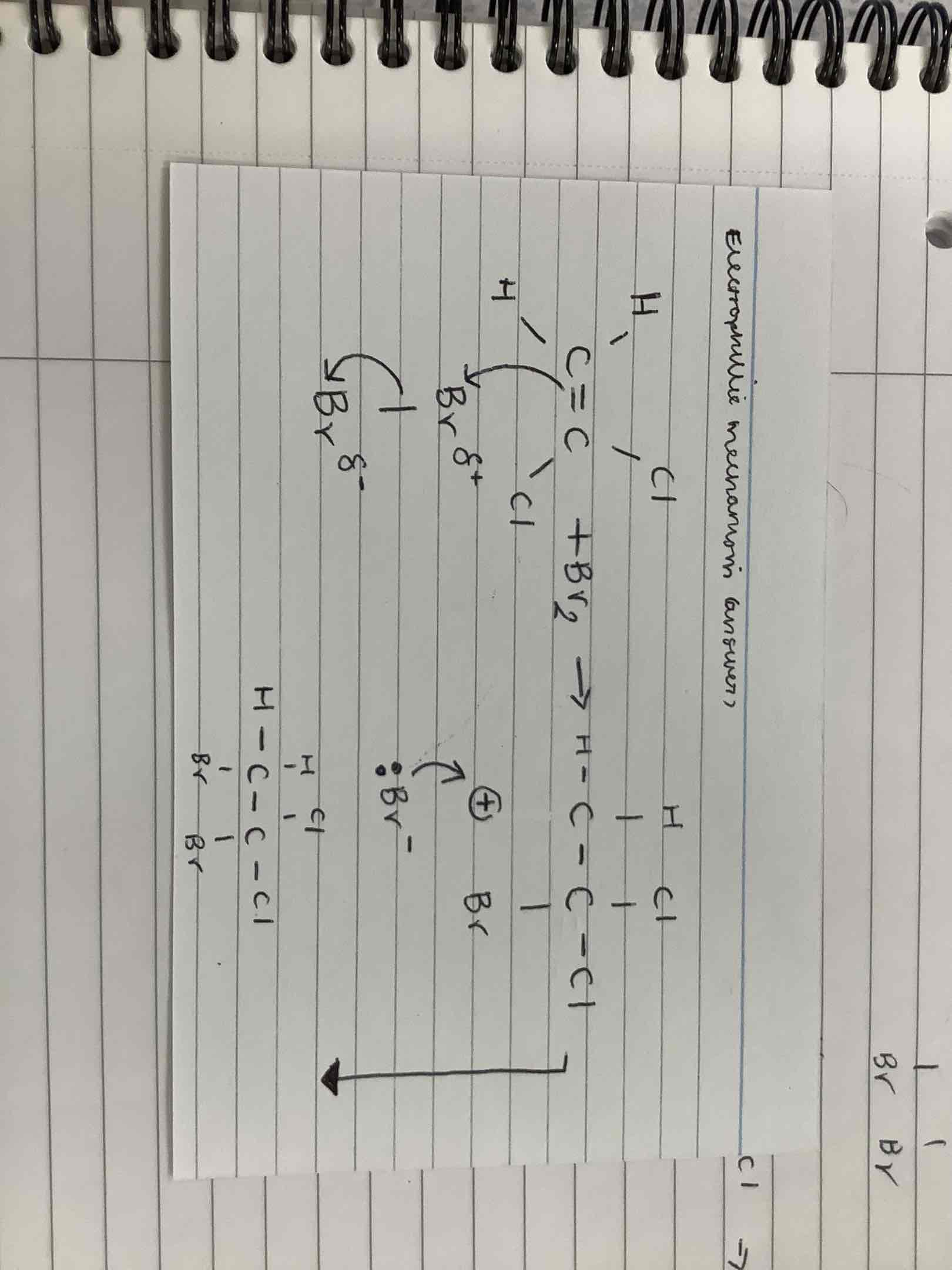
What is a polymer?
A large molecule formed from thousands of repeat units of smaller molecules known as monomers.
What is a monomer?
A small molecule that combines with many other monomers to form a polymer.
What is a repeat unit?
A specific arrangement of atoms that occurs in the structure over and over again
Repeat units are included in brackets where the symbol outside is n
What does a curly arrow represent?
The movement of TWO electrons.
What is a stereoisomer?
Same structural formulae but different arrangement of atoms in space.
When identifying the monomer that will give rise to a section of an addition polymer, will it always have a double bond? TRUE or FALSE
TRUE; this is because the double bond is the reactive site that breaks open during the polymerisation process to link monomers together without the loss of any small molecules.