Rates of reaction introduction
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
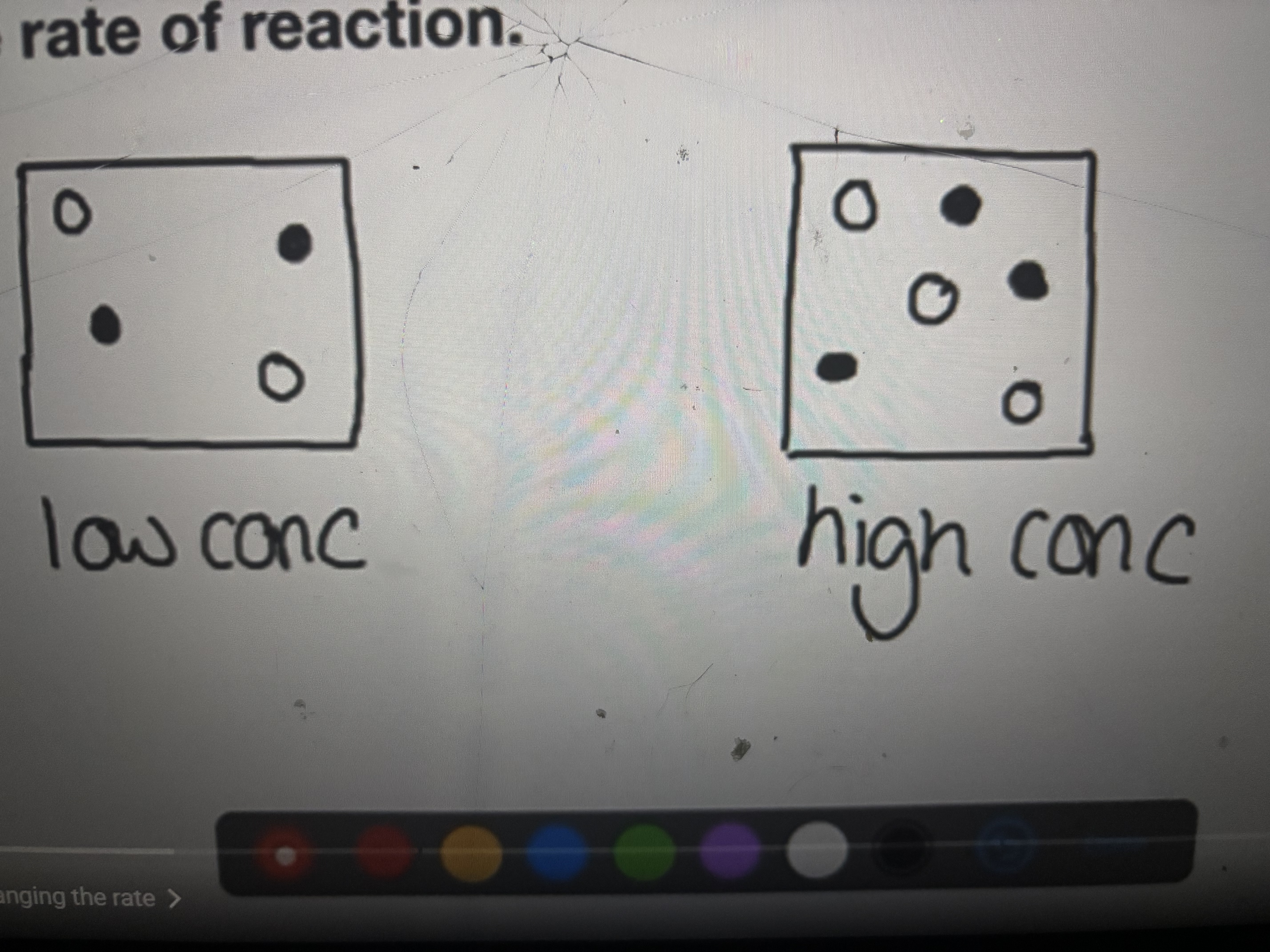
Increasing concentration
Increase the number of collisions in given volume, this will increase the frequency of collisions.
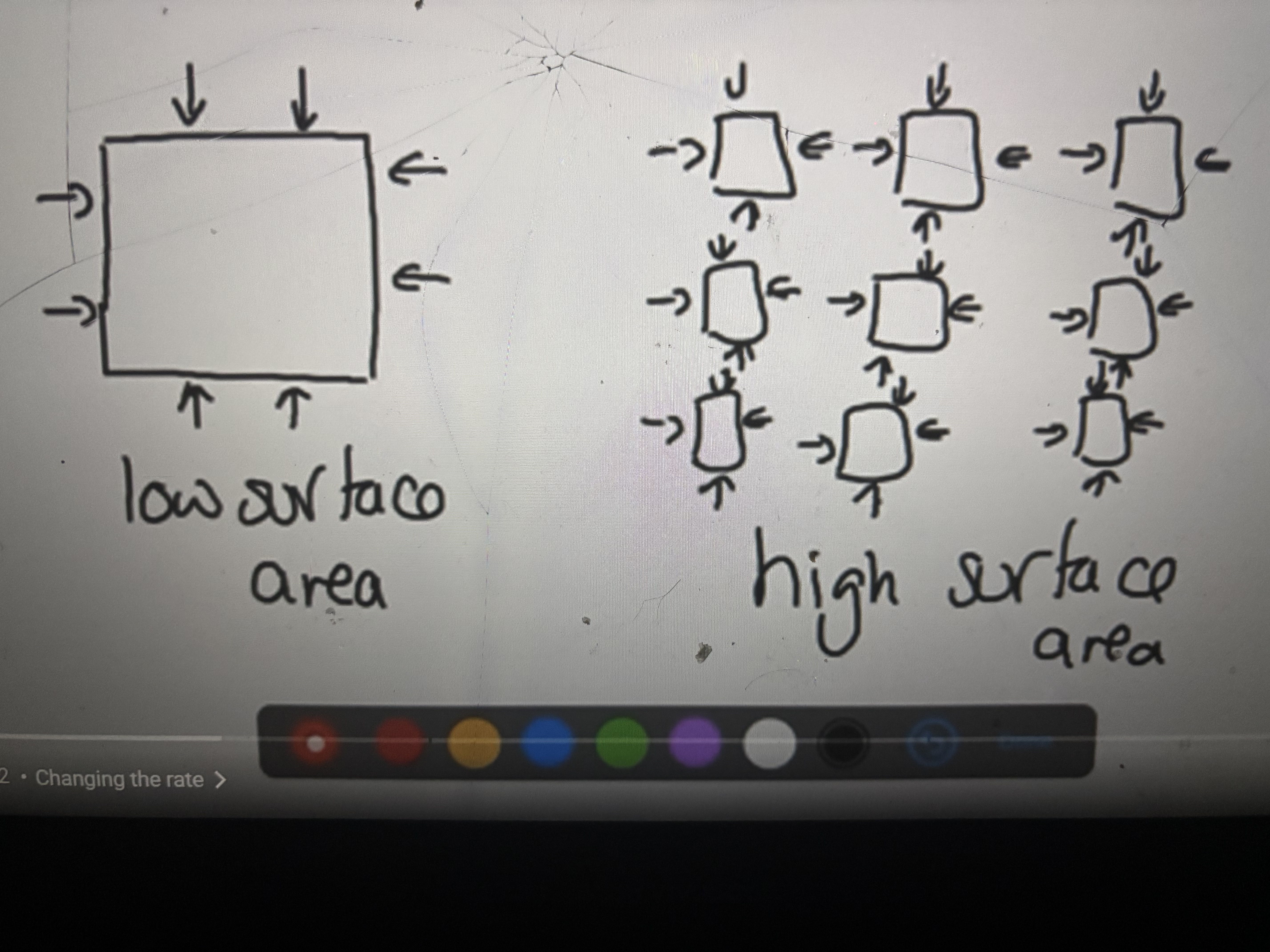
Increasing surface area
Reducing particle size will increase the frequency of collision
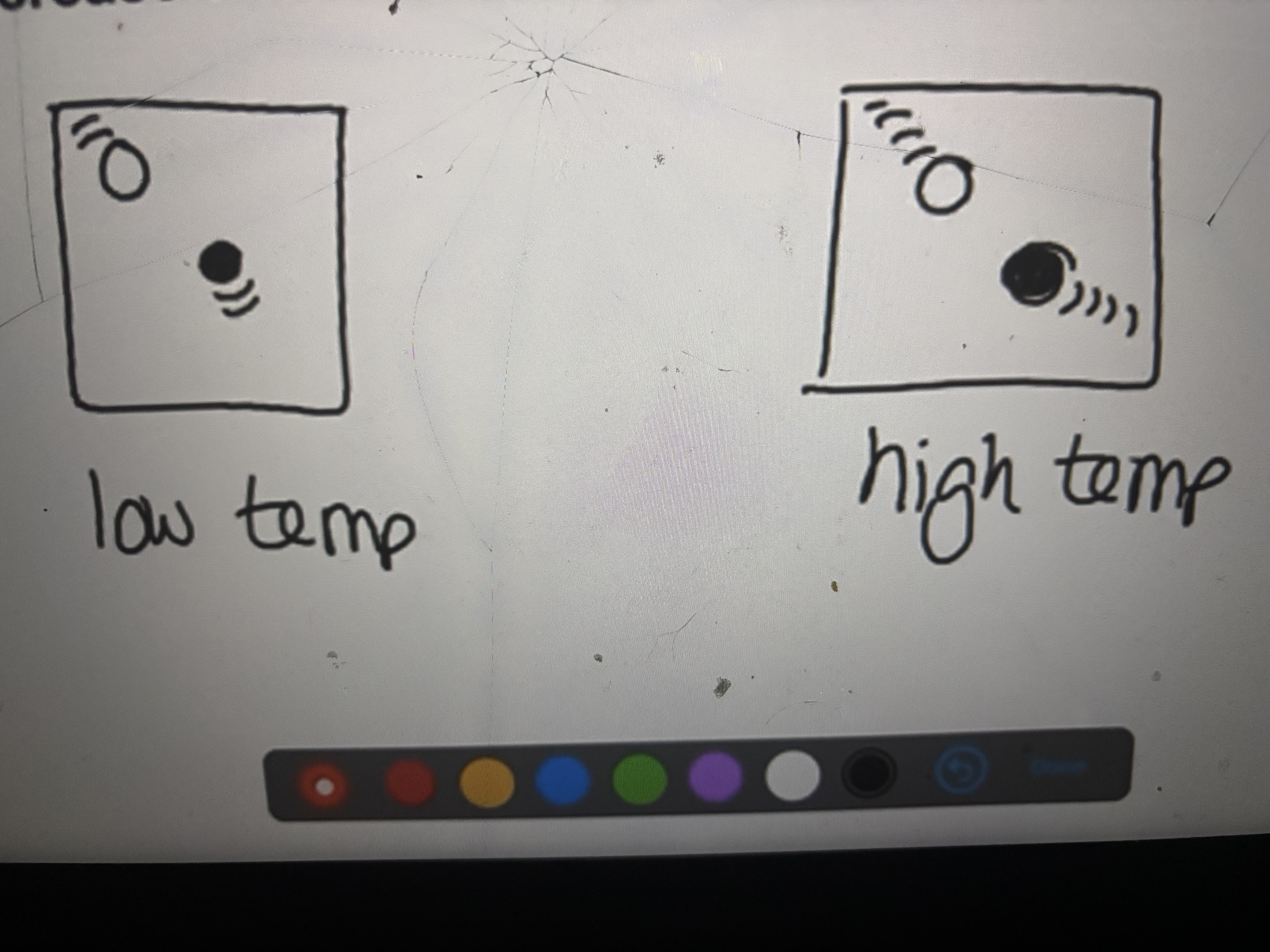
Increasing temperature
Increase the frequency and energy of collisions
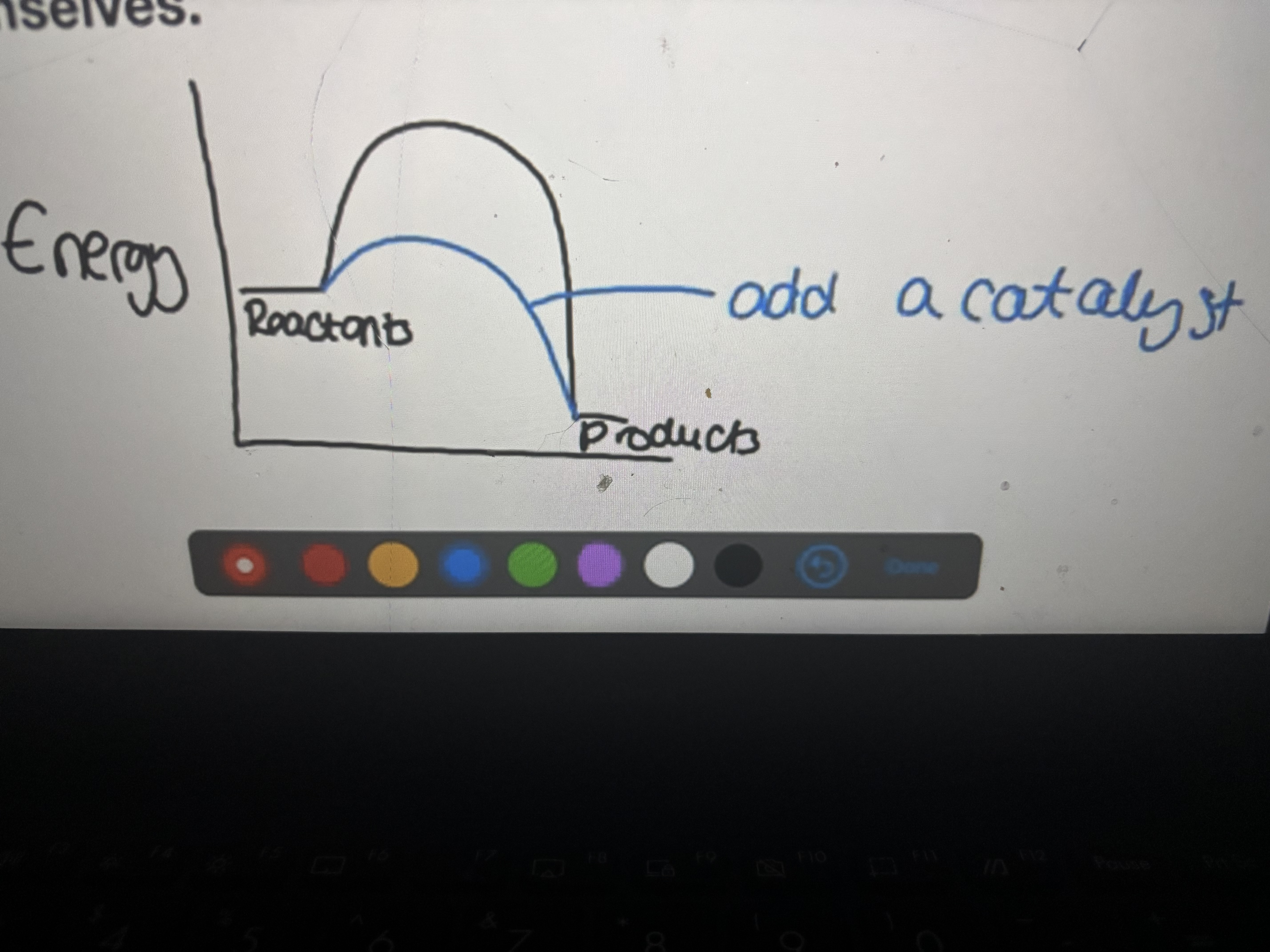
Adding a catalyst
Provide lower energy route for reaction therefore the reaction rate will increase
What does the term ‘Provide lower energy route’ mean?
Energy required for reaction is lowered
Follow the progress of a reaction
Change in mass, volume of gas
Where will the line be steepest
At the start of a reaction
Why will the line slow down
The reactants get used up
Where will the line become less steep
At the end of the reaction
When does the reaction stop
When all the reactant is used up and the line goes flat
Average rate equation
change in quantity/ change in time
Indicators of a chemical reaction?
Change in pH, change in temperature, effervescence, colour change and precipitation
The rate can be determined by measuring the changes in?
Concentration, mass and volume
How does temperature affect the rate of reaction?
Reactant particles gain more kinetic energy, move faster and collide with greater force
What are successful collisions
Collisions with enough energy to overcome the activation energy barrier and form products
How does concentration affect the rate of reaction?
There are more reactant particles in a given volume, meaning there are more opportunities for effective collisions to occur
How does particle size affect the rate of reaction?
Smaller solid particles have a larger surface area, exposing more reactant particles
What do collisions result in?
A chemical reaction
Variable
Something we can change during a chemical reaction
Rate
A measure of the change of a quantity over a period of time