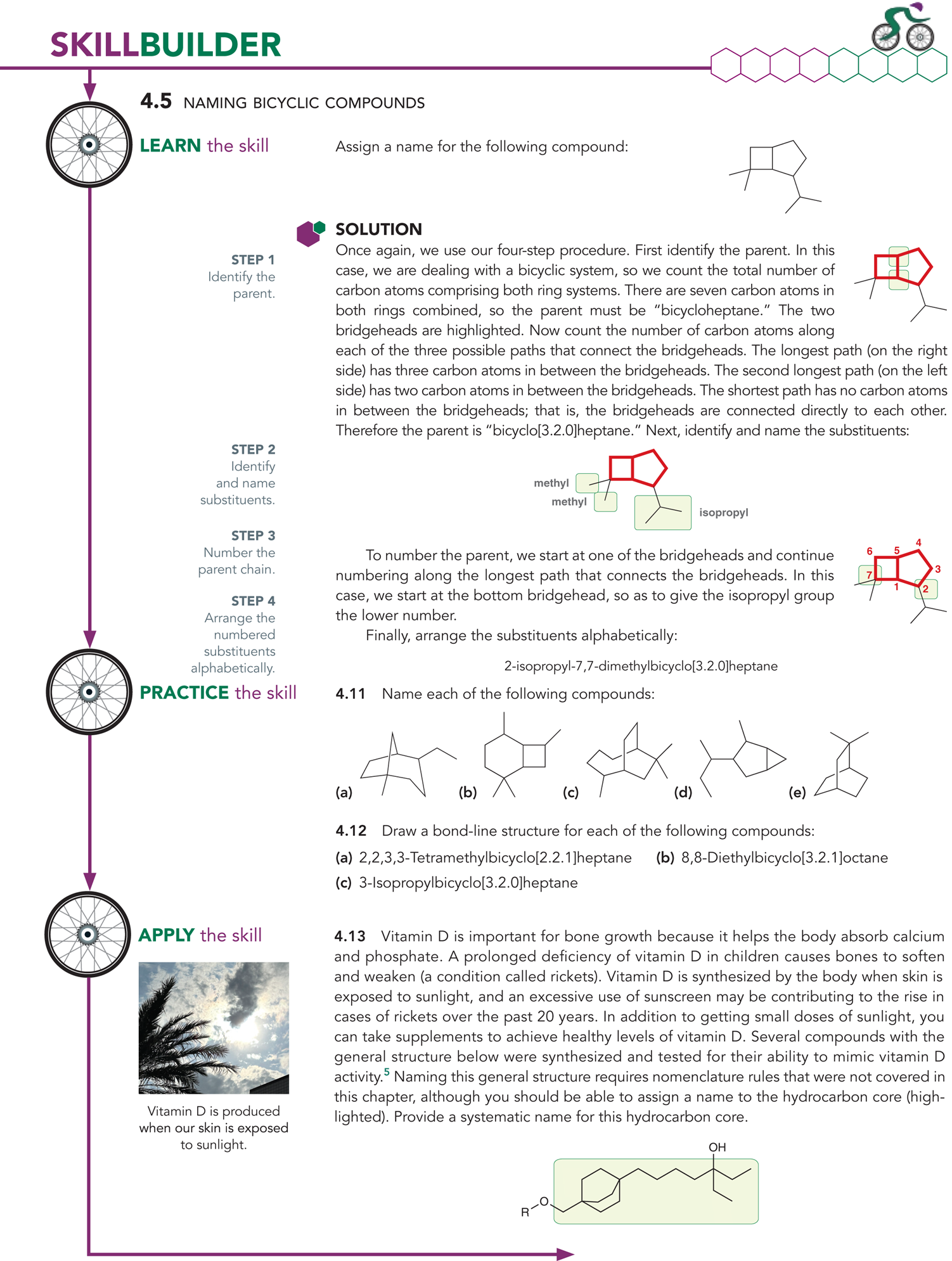
4.2 Nomenclature of Alkanes
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What are some organic compounds named after?
After a person, place, or thing
What are systemic names?
Names produced by IUPAC rules
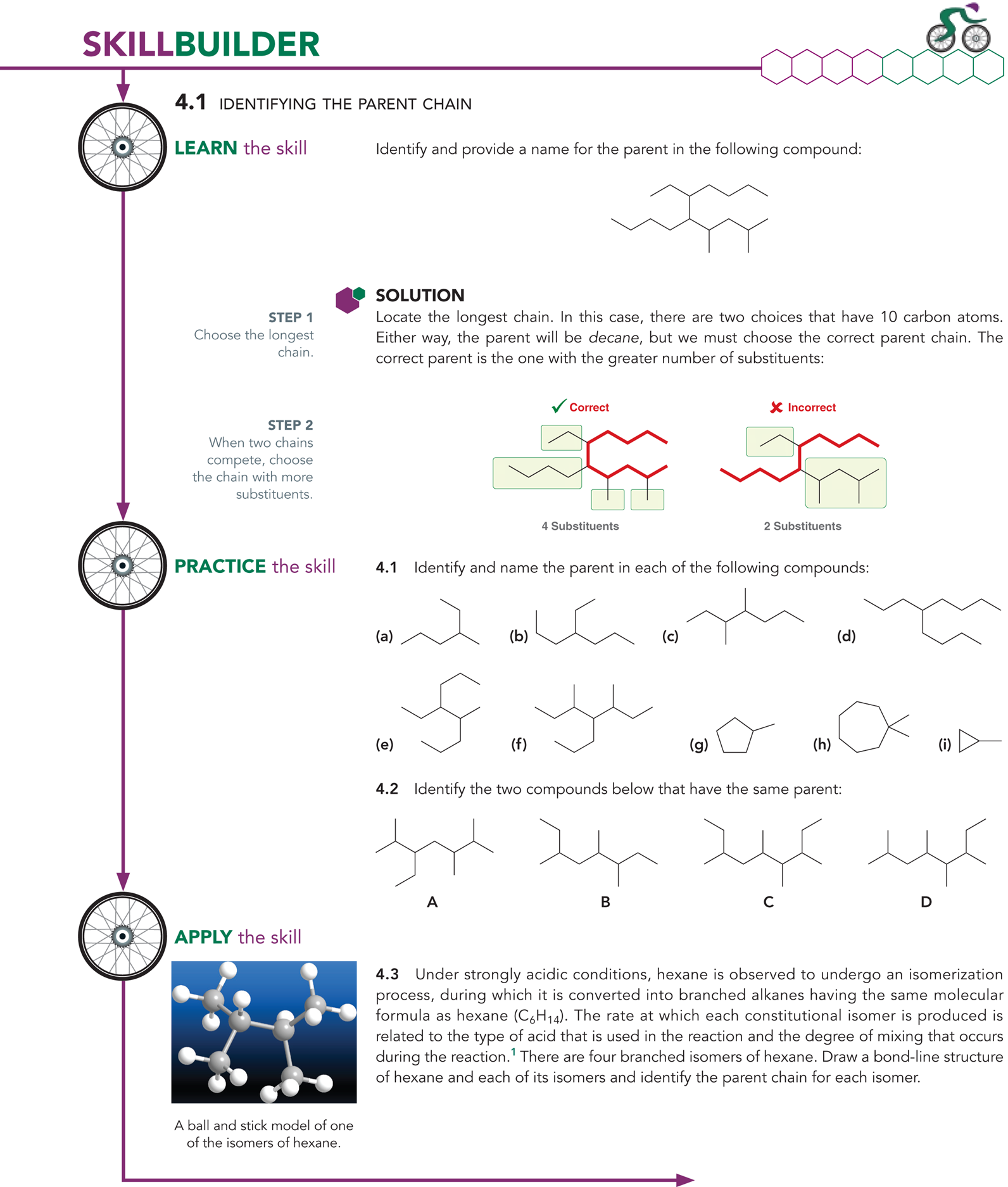
What is the first step in naming an alkane?
The first step is identifying the longest chain
Ex: You will look at the path that has the most amount of carbons
Ex: In the example picture, the longest parent chain has 9 carbons

What is a parent chain?
The longest chain in a compound
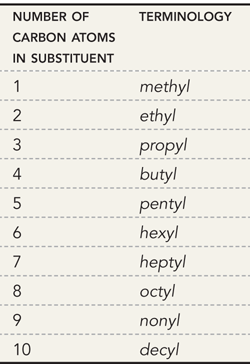
What are the parent names for alkanes (remember the first 10)
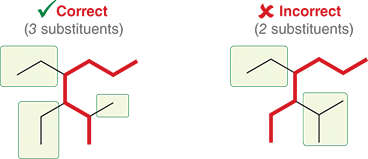
What happens if the parent chains are equal in length?
You will look at the chain with the greater number of substituents
What are substituents?
They are the branches connect to the parent chain
What are cycloalkanes?
Is used to indicate the presence of a ring in an alkane structure

Complete this practice sheet
What happens after you identify and name the parent chain?
List all of the substituents
Name them
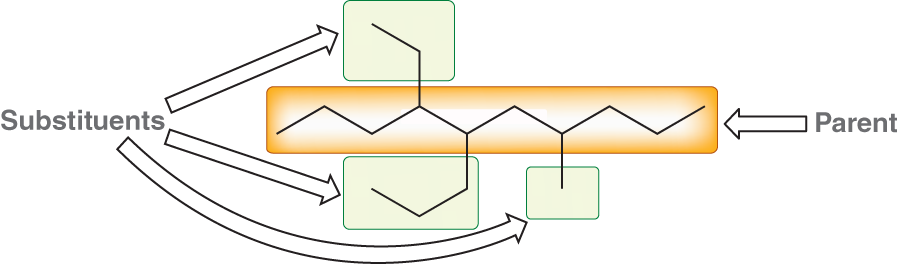
How do we name the substituents?
They are named with the same terminology used for naming parents only we add the letters “yl”
Memorize these names to name the substituents
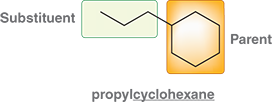
What happens when an alkyl group is connected to a ring?
The ring is generally treated as the parent
Ex:
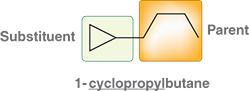
What happens when the alkyl group is connected to a ring comprised of fewer atoms then the rest of the structure?
The ring will be the substituent
Ex:
Do the following practice problems
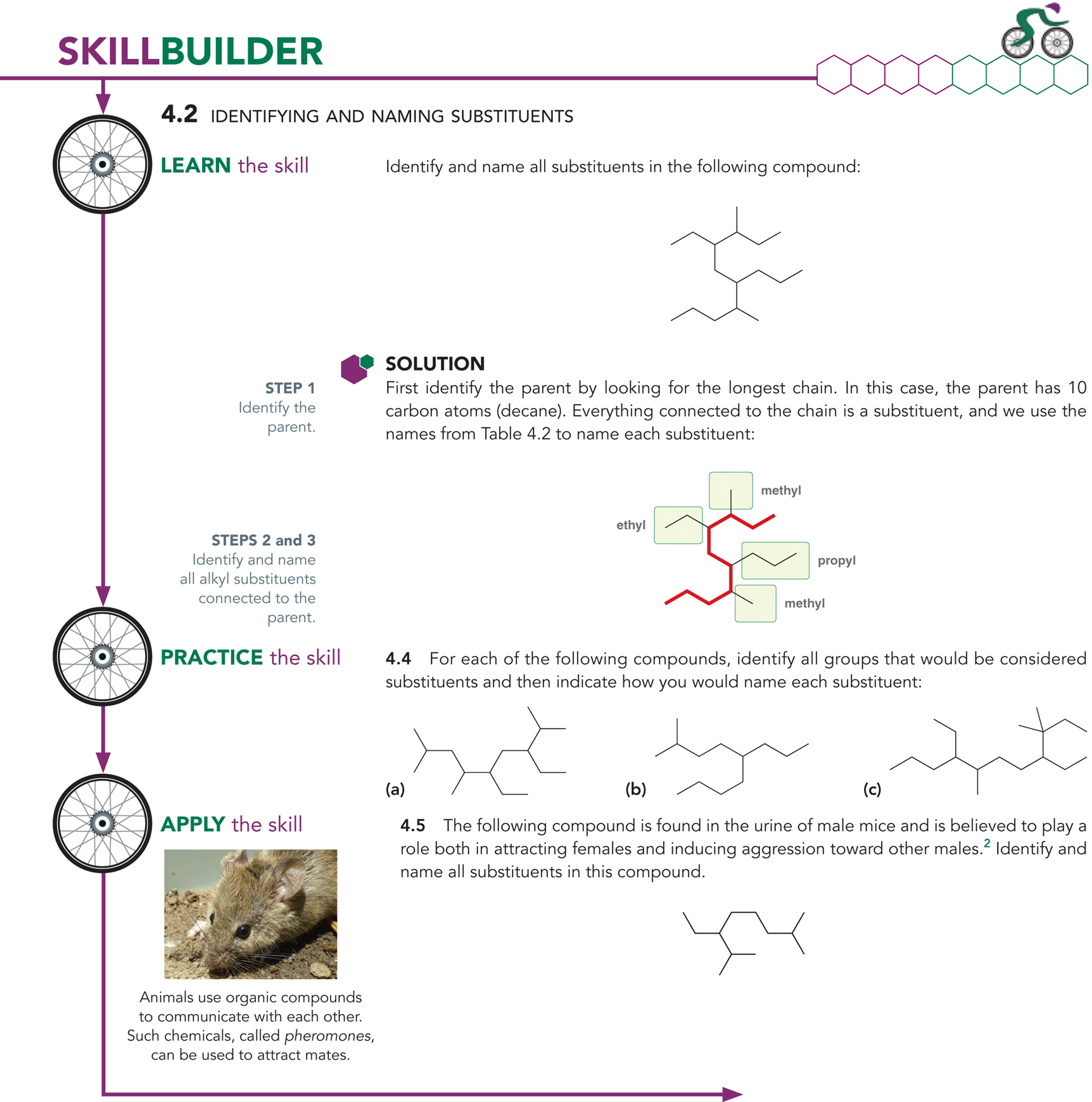
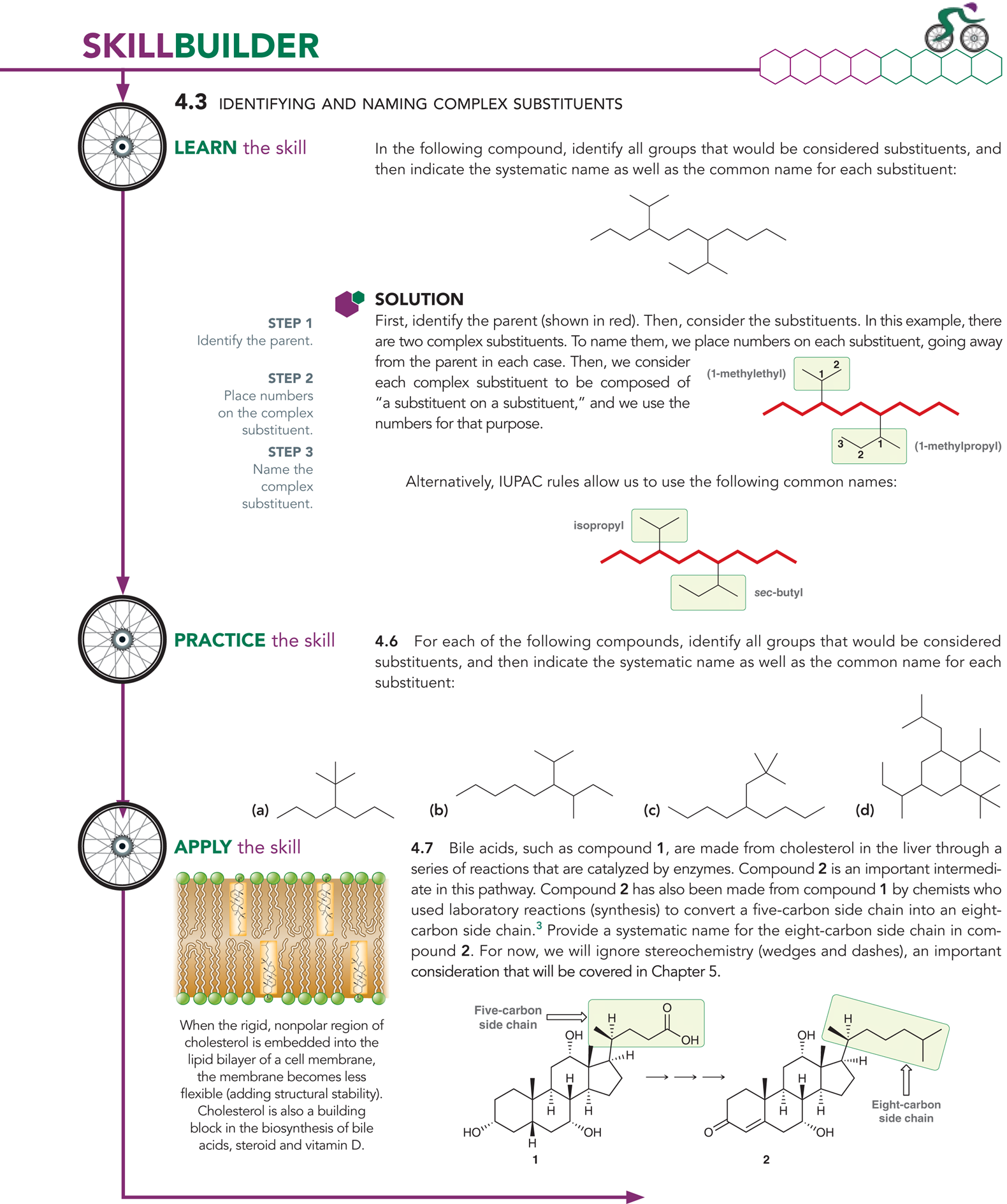
What happens when you have to name complex alkyl substituents?
You will begin by placing numbers on the substituent, going away from the parent chain
You will name them using a list
Ex:
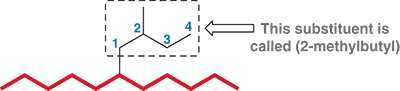
What is the name for an alkyl group with 3 carbon atoms
Propyl
Isopropyl
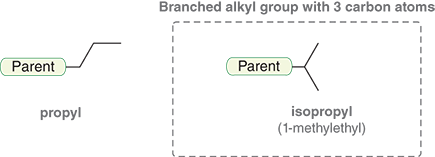
What is an alkyl group with four carbons named
Butyl
Sec-butyl
Isobutyl
Tert-butyl
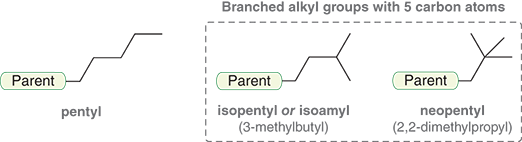
What are alkyl groups with five carbons named?
Pentyl
Isopentyl
Neopentyl
Answer the following practice problem questions
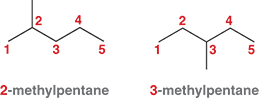
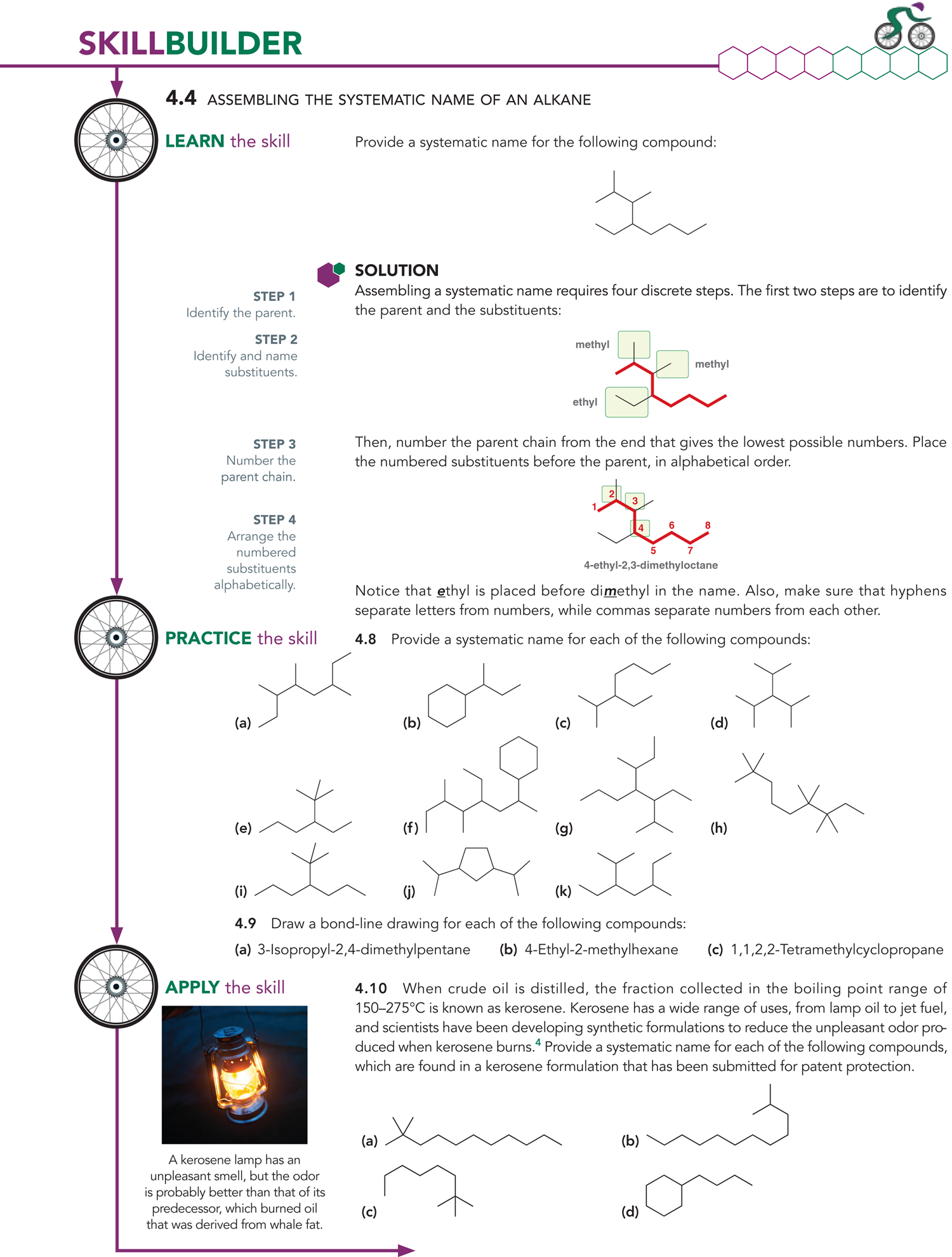
How do we assemble the systematic name of an alkane?
To name an alkane, first number the carbons in the main chain. Then, use those numbers to show where each branch (side group) is attached.
Ex:
What is a locant?
a number used to identify the location of a substituent
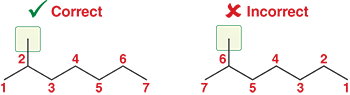
How do you decide where to start numbering the parent chain in IUPAC naming?
Start numbering from the end closest to the substituent. This gives the substituent(s) the lowest possible number(s), called locants
Ex: In this example, we placed the numbers so that the methyl is at C2 rather than C6
Ex: In this following case, we number the parent chain so that the substituents are 2,5,5 rather than 3,3,6 because we want the first number to be as low as possible
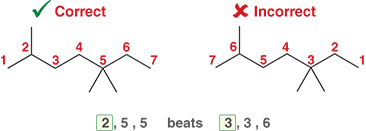
What happens if there is a tie for the first number?
The second number should be as low as possible
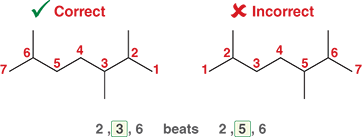
What happens if the second number still does not break the tie
Then we assign the lower number to the group that comes first alphabetically
What happens when you are naming a cycloalkane with only one substituent connected to the ring?
The substituent is understood as C1, therefore, no number is used when naming such a monosubstituted cycloalkane

What happens when you are naming a cycloalkane with more than one substituent?
Each substituent requires a number to identify the location
The goal is to have the lowest possible set of numbers
Ex:
What do you do when a substituent appears more than once in a compound?
A prefix is used to identify the compound
di-2
tri=3
tetra=4
penta=5
hexa=6
Use a hyphen to separate numbers from letters
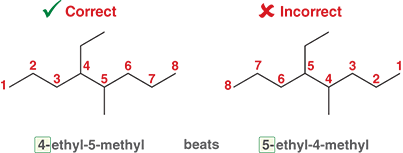
What do you do at the end when once all substituents have been identified and assigned the proper numbers?
They are placed in alphabetical order
Pre-fixes are not included in the alphabetization
Practice these practice questions
What is a bicycloalkane?
It is an alkane with two rings that share carbon atoms. It is like a cycloalkane, but with two connected rings instead of one
Ex:

in bicycloalkanes, what extra information does the parent name need to show?
It must show how the two rings are connected. This is done by identifying the two bridgehead carbons.
What are bridgehead carbons?
Are the two carbon atoms where the rings are joined together
Ex:
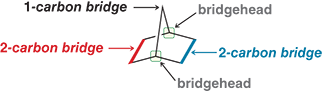
How do you assign the numbers inside the brackets when naming a bicycloalkane?
Find the two bridgehead carbons. Then count the number of carbons (excluding the bridge heads) along each of the three paths connecting them. Write these numbers from largest to smallest inside the brackets
Ex: [2.2.1]
![<ol><li><p>Find the two bridgehead carbons. Then count the number of carbons (excluding the bridge heads) along each of the three paths connecting them. Write these numbers from largest to smallest inside the brackets</p></li><li><p>Ex: [2.2.1]</p></li></ol><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3246e474-b07c-476a-bb4d-0898a41e6088.png)
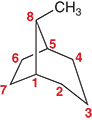
How do you number the parent chain a bicycloalkane
Start at one of the bridgehead and number along the longest path first, then the second longest path, then finally the shortest path. This ensures that substituents get the correct locants.
Ex:
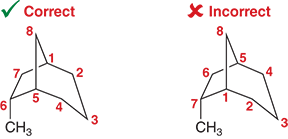
Explain the image
In this example, it is not possible to assign a low number to the methyl substituent. It ended up with a high number because of its location at the “end” of the numbered path taken. Specifically, it is on the shortest path connecting the bridgeheads. The shortest path is numbered last, so the methyl group had to be on C8. Regardless of the position of substituents, the parent must be numbered beginning with the longest path first. The only choice is which bridgehead will be counted as C1; for example:Either way, the numbers begin along the longest path. However, we must start numbering at the bridgehead that gives the substituent the lowest possible number. In the example above, the correct path places the substituent at C6 rather than at C7, so this compound is 6-methylbicyclo[3.2.1]octane.
Answer these practice problems