poo
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Gas particles are of negligible size compared to the space between them
Gases can be compressed
The particles are in continuous, rapid, random motion
Gas molecules moving randomly will collide and create pressure
Their collisions are elastic
Kinetic energy is conserved
There are no significant interactions among the particles of a gas (IMF)
No forces between molecules, attract or repell, allows gas to expand and fill throughout container
When temperature increases for a gas, the kinetic energy of the particles increases
Average kinetic energy is proportional to temperature, meaning faster molecules at higher temperature (therefore higher pressure)
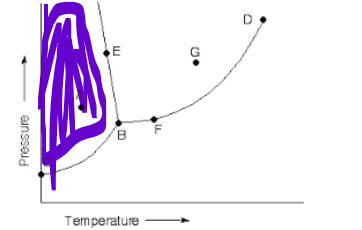
which state of matter
solid
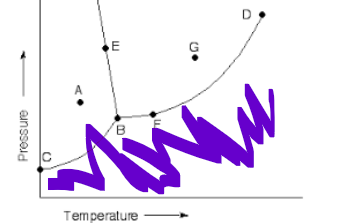
which state of matter
liquid
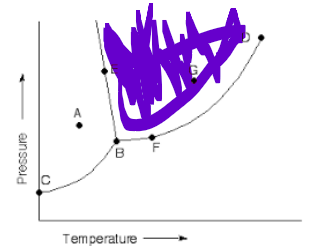
which state
gas
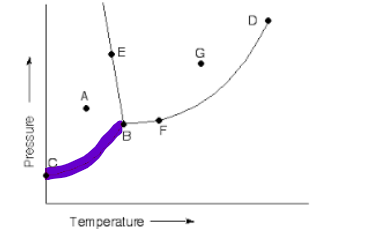
what is this line
sublime/deposit
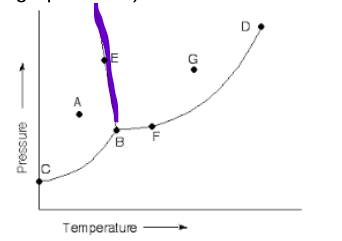
what is this line
melt/freeze
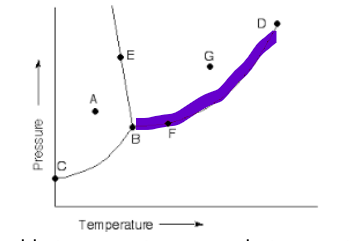
and this line
boil/condense