BIPN 100 Lecture #2 Terms - Nervous System Physiology II: Passive Membrane Properties
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Semipermiable
ions must cross membrane via ion channels
Ions
molecules w/ a net electrical charge
Ion Channels
membrane proteins w/ selective permeability for particular ions
Leak Channels
pores that remain open
Voltage-gated ion channels
open/close in response to changes in membrane potential
Ligand-gated ion channels
open in response to chemical signals binding
Membrane Potential (Vm)
difference in electrical charge between inside and outside of cell
voltage measured as relative differences between in/outside
Electrophysiology
method to measure membrane potential
Vm = V inside - V outside
a recording electrode is inserted into neuron
Resting Membrane Potential (RMP, V rest)
Vm when a cell is at rest, not firing an action potential
steady state: no net movement of charge
Around -50 to -70 mV
Sodium-potassium pump
3 Na+ out, 2 K+ in
builds up Na+ in ECF, K+ in ICF
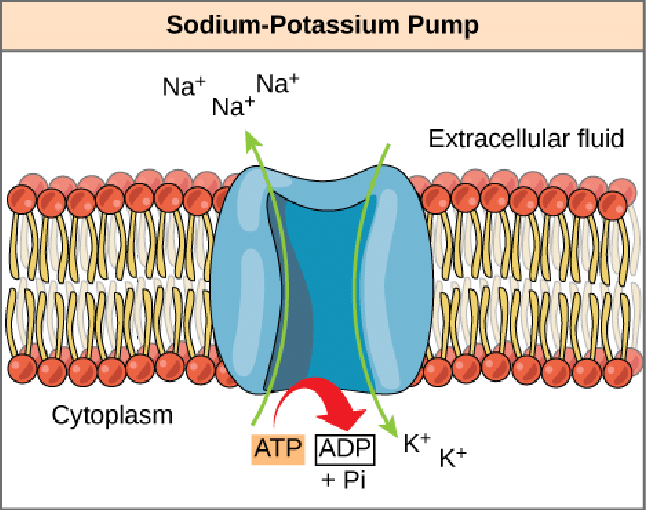
Antiport
carrier protein that moves substances in opposite directions
Active Transport
Na+-K+-ATPase hydrolyzes ATP to move ions against concentration gradient
What are 2 factors that determine Vm
Electrochemical Gradient
Differences in membrane permeability— ability for ions to pass through membrane
Electrochemical Gradient
uneven distribution of ions across cell membrane
Electrical Driving Force
Attraction and repulsion between charged particles
Chemical Driving Force
diffusion, drives ions from region of high to low
At rest, what is the membrane more permeable to and why?
The membrane is more permeable to K+ because #K+ leak channels > #Na leak channels
Equilibrium Potential (E ion)
membrane potential that exactly opposes concentration gradient
electrical & chemical forces are equal and opposite
no net movement of ions
Nernst Equation
Calculates equilibrium potential for a membrane permeable to one ion
z = ion charge
[ion] out = concentration of ion in ECF
[ion] in = concentration of ion in ICF
![<p>Calculates equilibrium potential for a membrane permeable to one ion </p><ul><li><p>z = ion charge</p></li><li><p>[ion] out = concentration of ion in ECF</p></li><li><p>[ion] in = concentration of ion in ICF</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/6aa7a208-d253-400d-9736-8015f630236e.png)
Calculating F ion
Driving forces on an ion are dependent on the difference between Vm and E ion
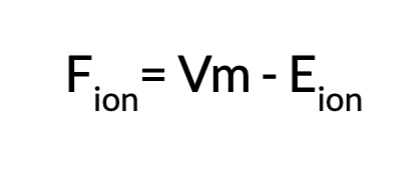
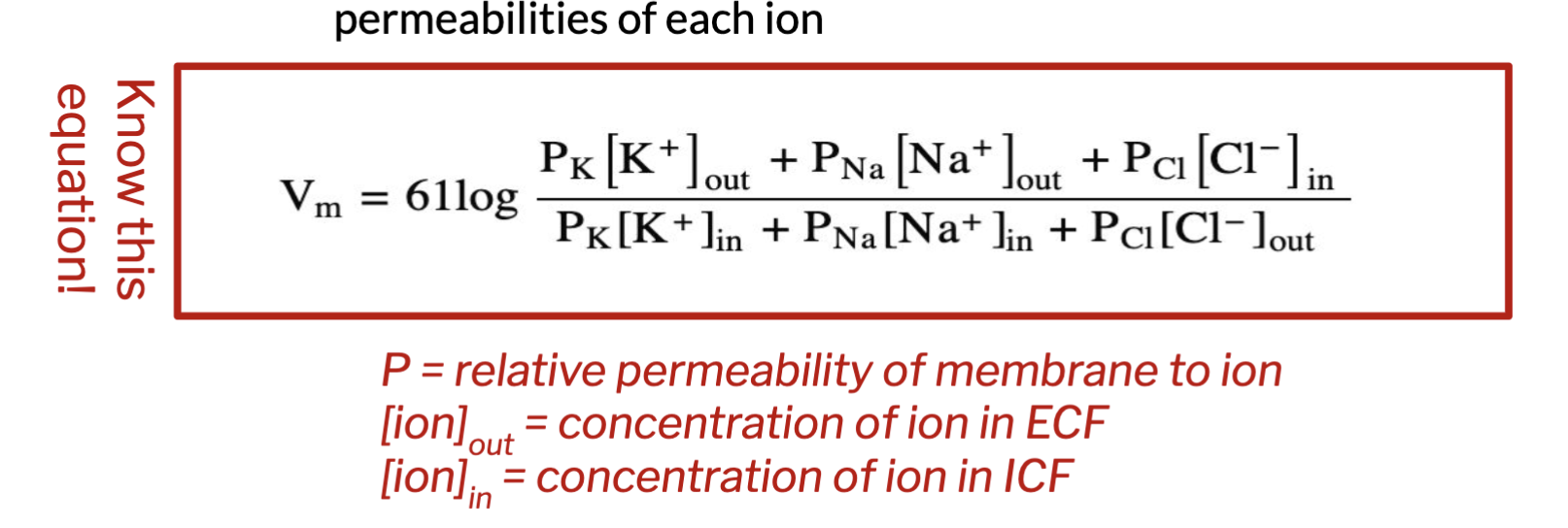
Goldman-Hodgkin-Katz (GHK) Equation
calculates membrane potential resulting from contribution of E ions of all ions as a function of permeability
permeability = ion contribution to membrane potential
Electrical Current (I)
flow of electrical charge carried by an ion
measured in amperes (amps)
ion movement produces electrical signals
I dependent on F ion and permeability
Conductance (G)
ease with which ions flow across membrane
units: siemens
conductance determined by # open ion channels
stimuli alter permeability —> ions flow w/ electrochemical forces
Resistance (R)
force that opposes flow
inverse of conductance (1/G)
Units: Ohms (omega)
in neurons:
membrane resistance (Rm)
cytoplasm resistance (Ri)
Ohm’s Law
states that current flow is directly proportional to electrical potential difference between 2 points and conductance
small changes in [ion] —> big changes to membrane potential
![<p>states that current flow is directly proportional to electrical potential difference between 2 points and conductance </p><ul><li><p>small changes in [ion] —> big changes to membrane potential </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/2e08d9d8-97c4-44a9-b217-8970ef074f09.png)
Depolarization
increase in Vm
membrane becomes more permeable to Na+
inward I Na+ w/ electrochemical gradient
Vm above RMP
Hyperpolarization
decrease in Vm
membrane becomes more permeable to K+
outward I K+ w/ electrochemical gradient or inward I Cl-
Vm below RMP