CEM 252 - Aromatic compounds
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
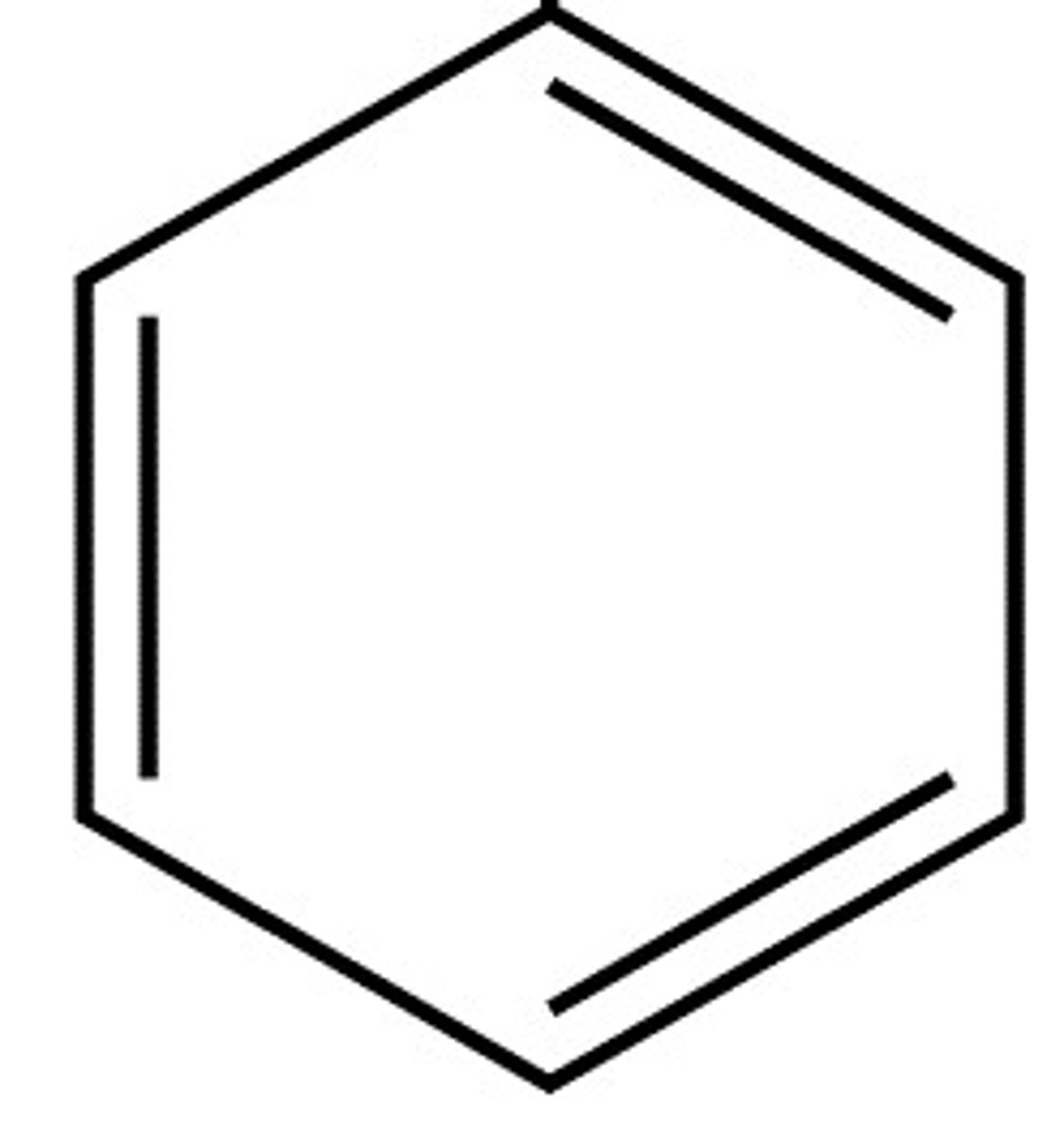
What is benzene?
Double bonded ring
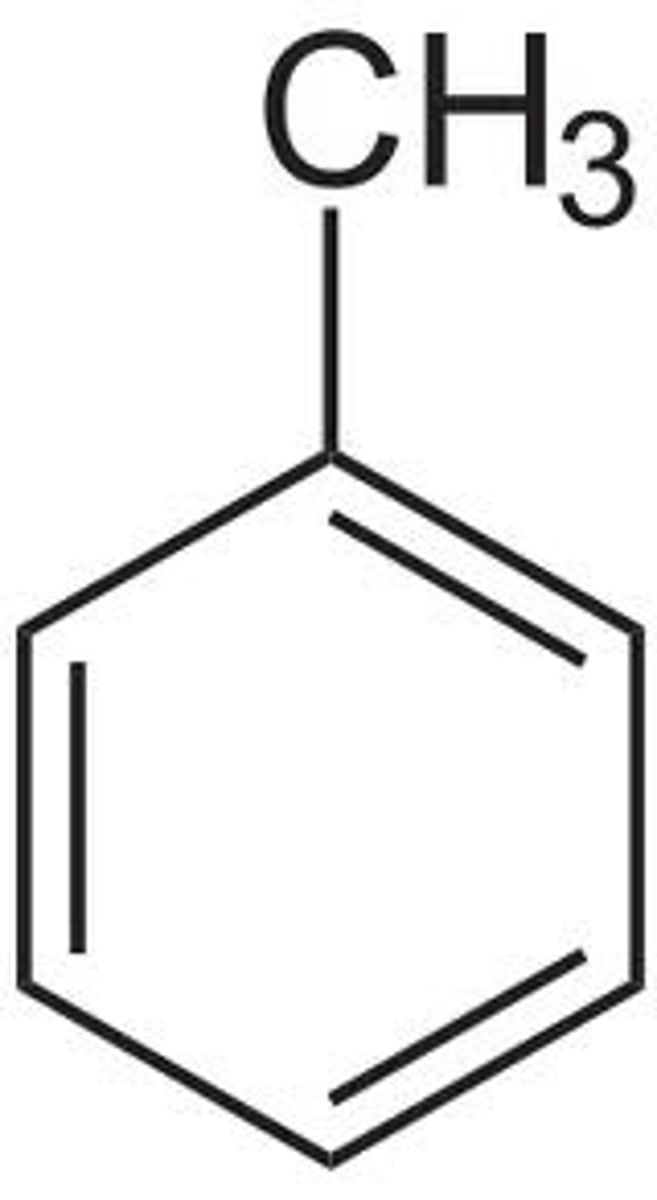
What is toluene?
Benzene ring with a methyl group attached
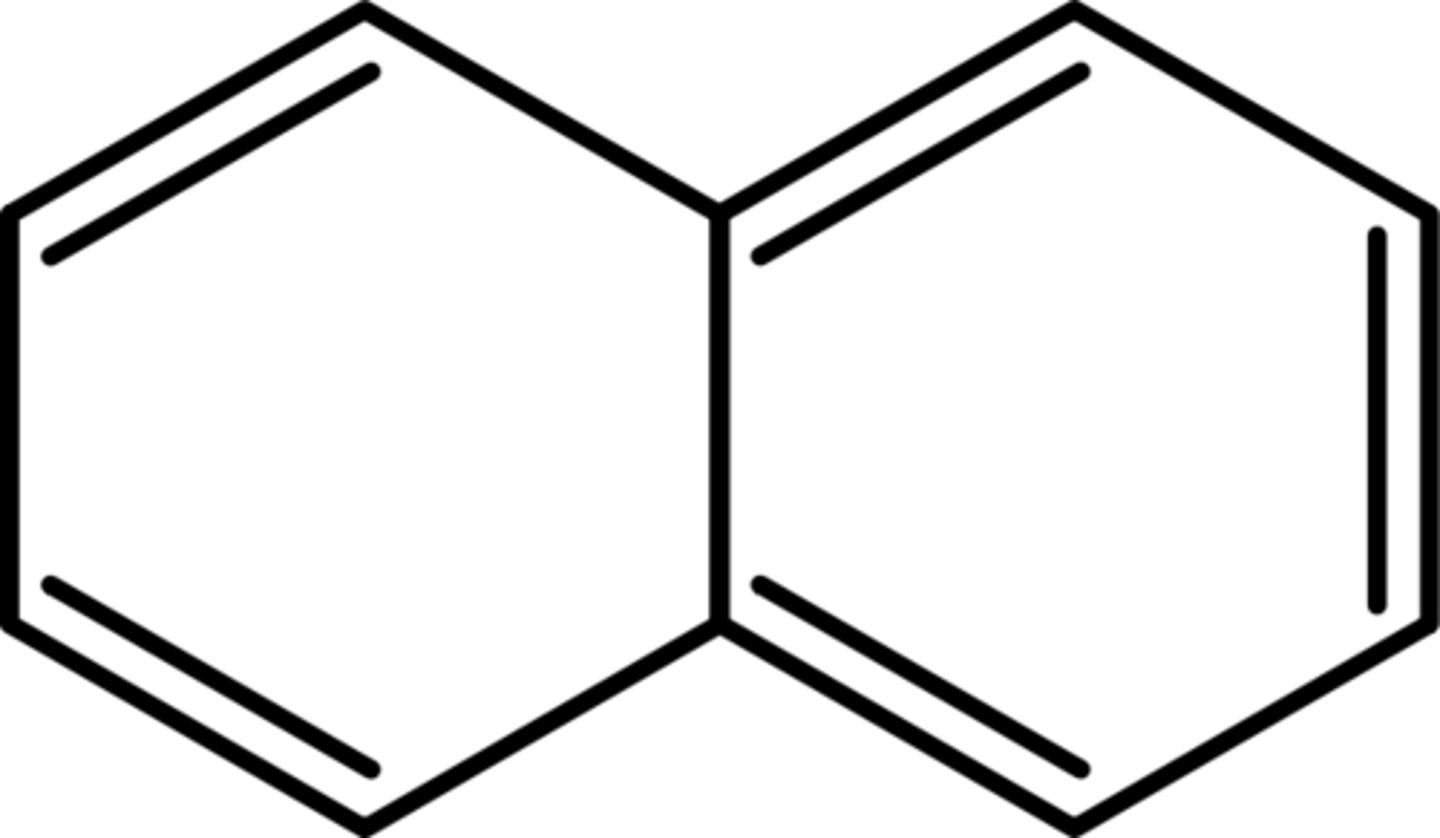
What is napthalene?
Two double bonded rings connected
True or false: Benzene does not have addition reactions?
True
What does benzene need for an addition reaction?
A catalyst
What are the 3 rules for determining if a molecule is aromatic or not?
1. The compound needs to be cyclic and planar
2. All atoms of the ring need to be conjugated (need p-orbitals)
3. The compound is only allowed to have 4n + 2 aromatic electrons (2, 6, 10, 14)
Which electrons do you count when determining aromaticity?
The pi-bond electrons ONLY
If a compound follows the first 2 rules of aromaticity and has 4n number of pi-electrons (4, 8, 12, 16), then what is it considered to be?
Anti-aromatic
Can radicals be aromatic?
No, because they have an extra electron
Can molecules with charges be aromatic?
If the charges add up to the correct number of electrons to follow the aromaticity rules
When a heteroatom is in a ring with a double bond, do the electrons get counted?
No
When a heteroatom is in a ring with a single bond, do the electrons get counted?
Yes, but only count one pair of lone pairs
What are the rules for naming aromatic compounds?
-"Benzene" will act as the parent chain
-One side group is named in front of the name benzene
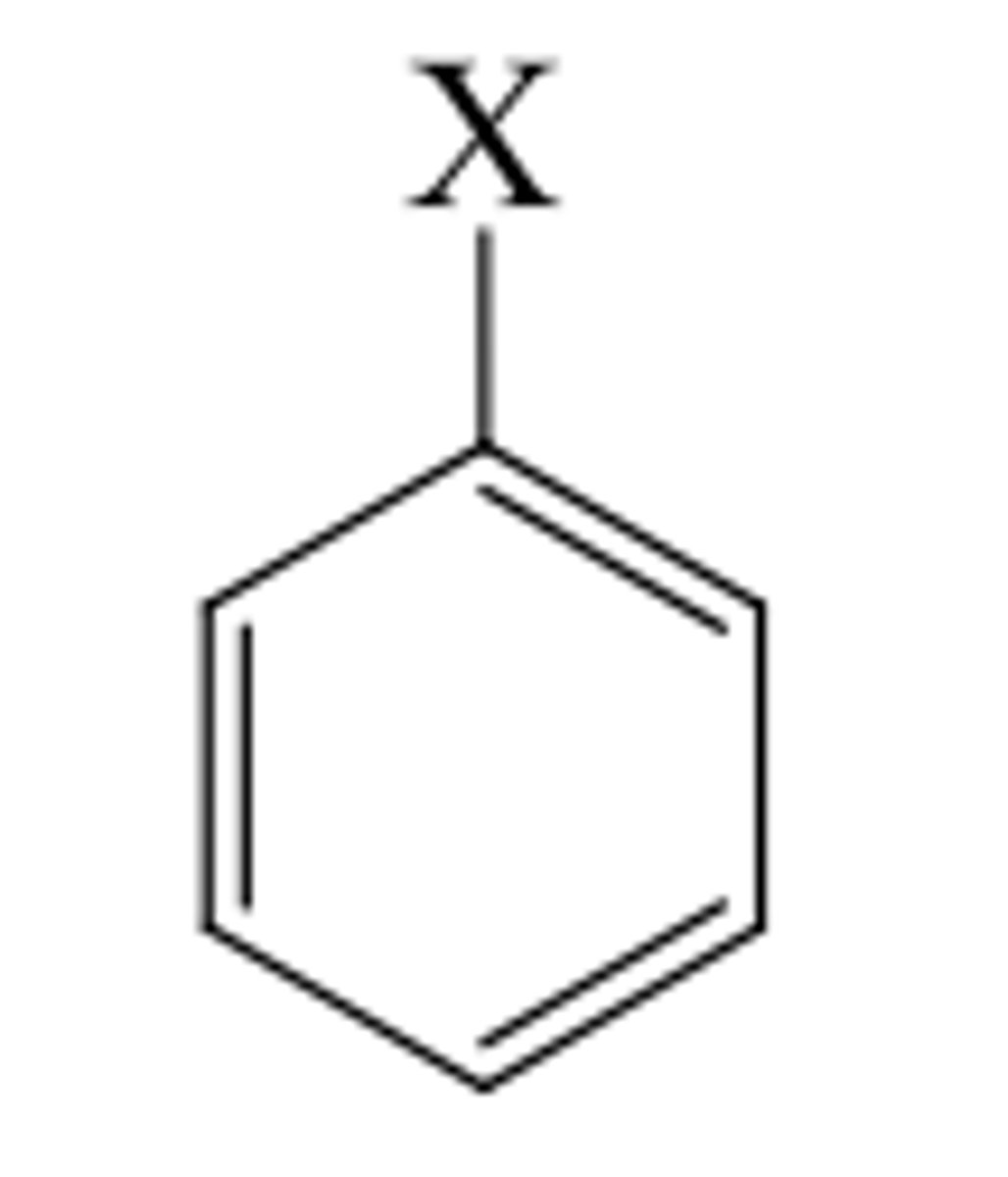
What is halobenzene?
A benzene ring with a halogen coming off it
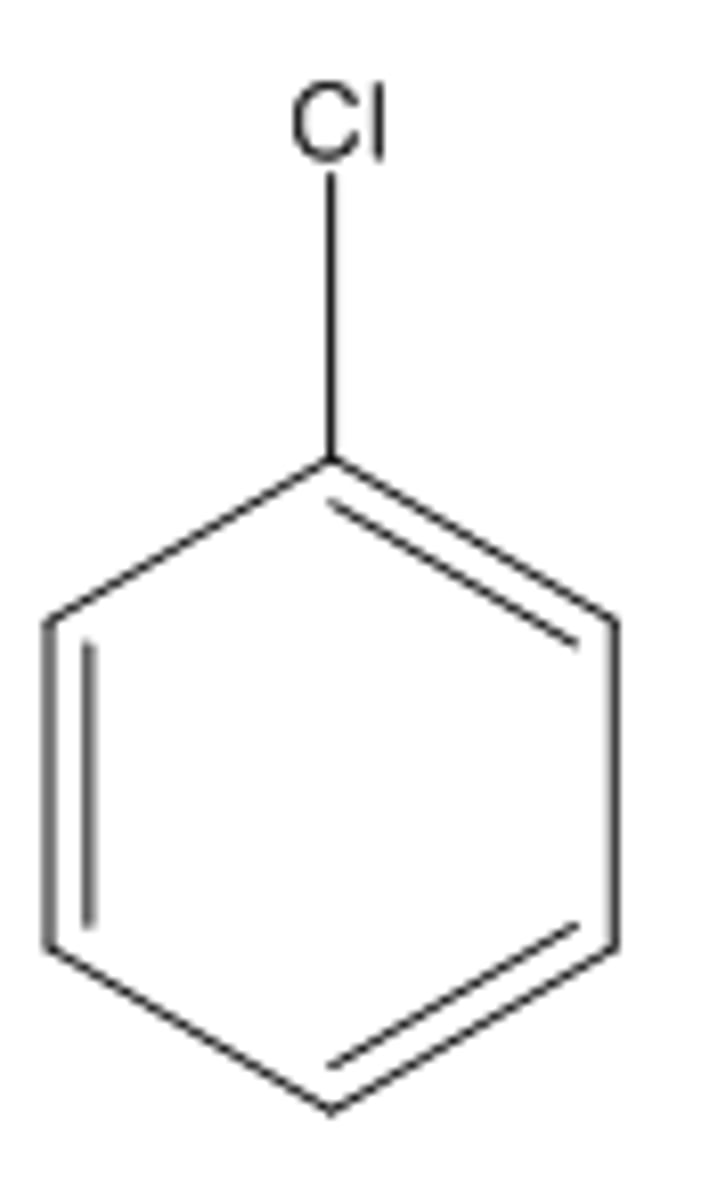
What is chlorobenzene?
A benzene ring with a chlorine group coming off it
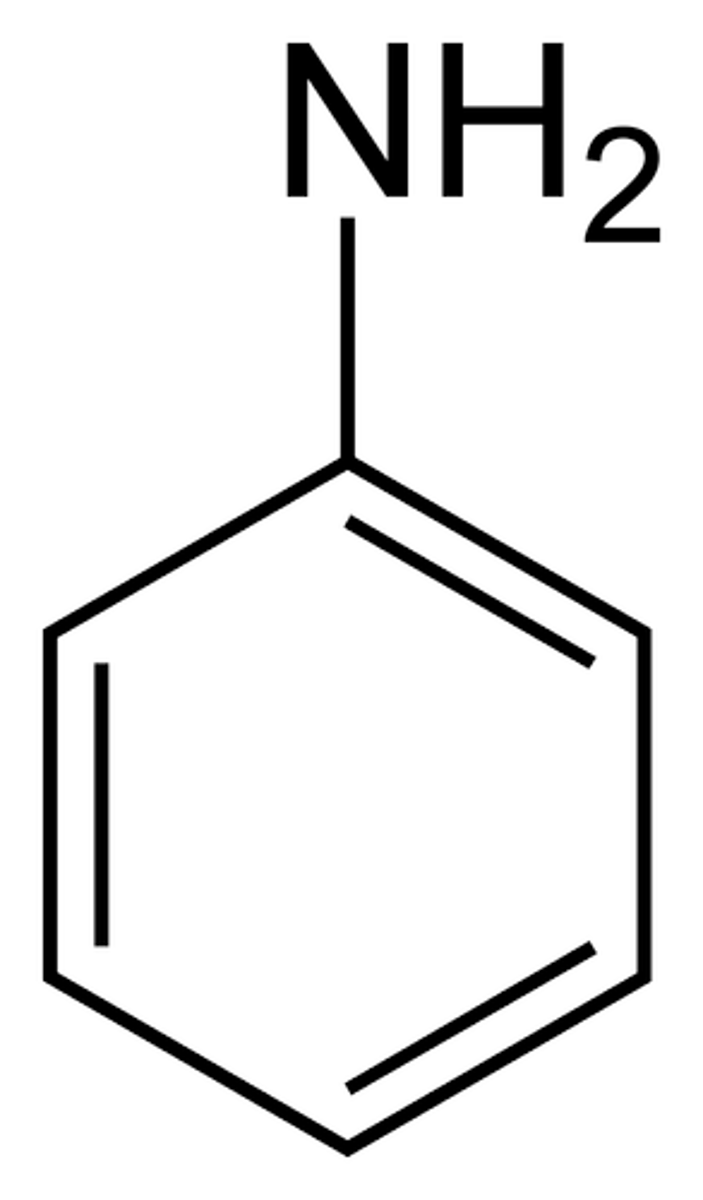
What is aminobenzene?
A benzene ring with an amino group (NH2) coming off it
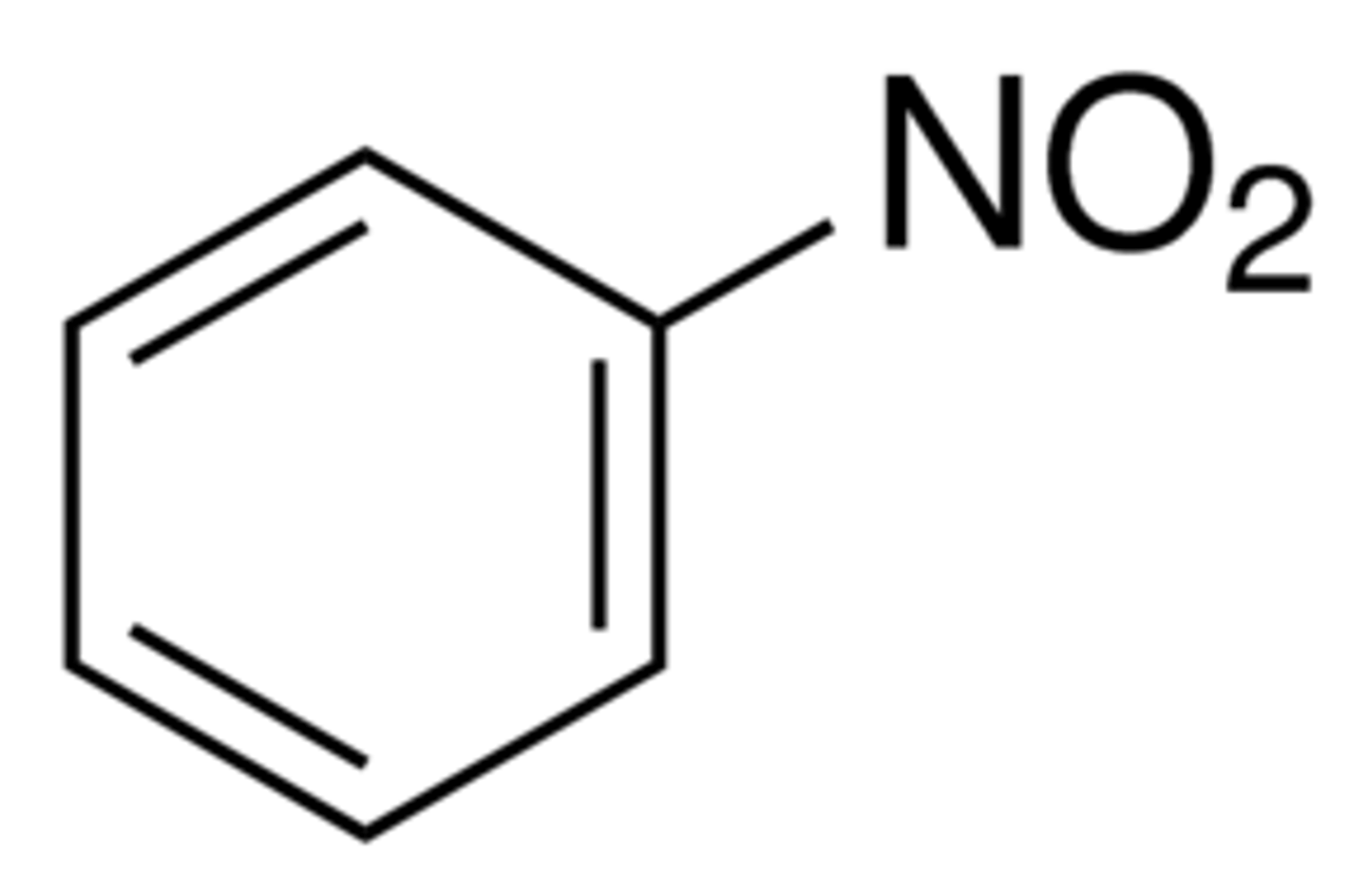
What is nitrobenzene?
A benzene ring with a nitro group (NO2) coming off it
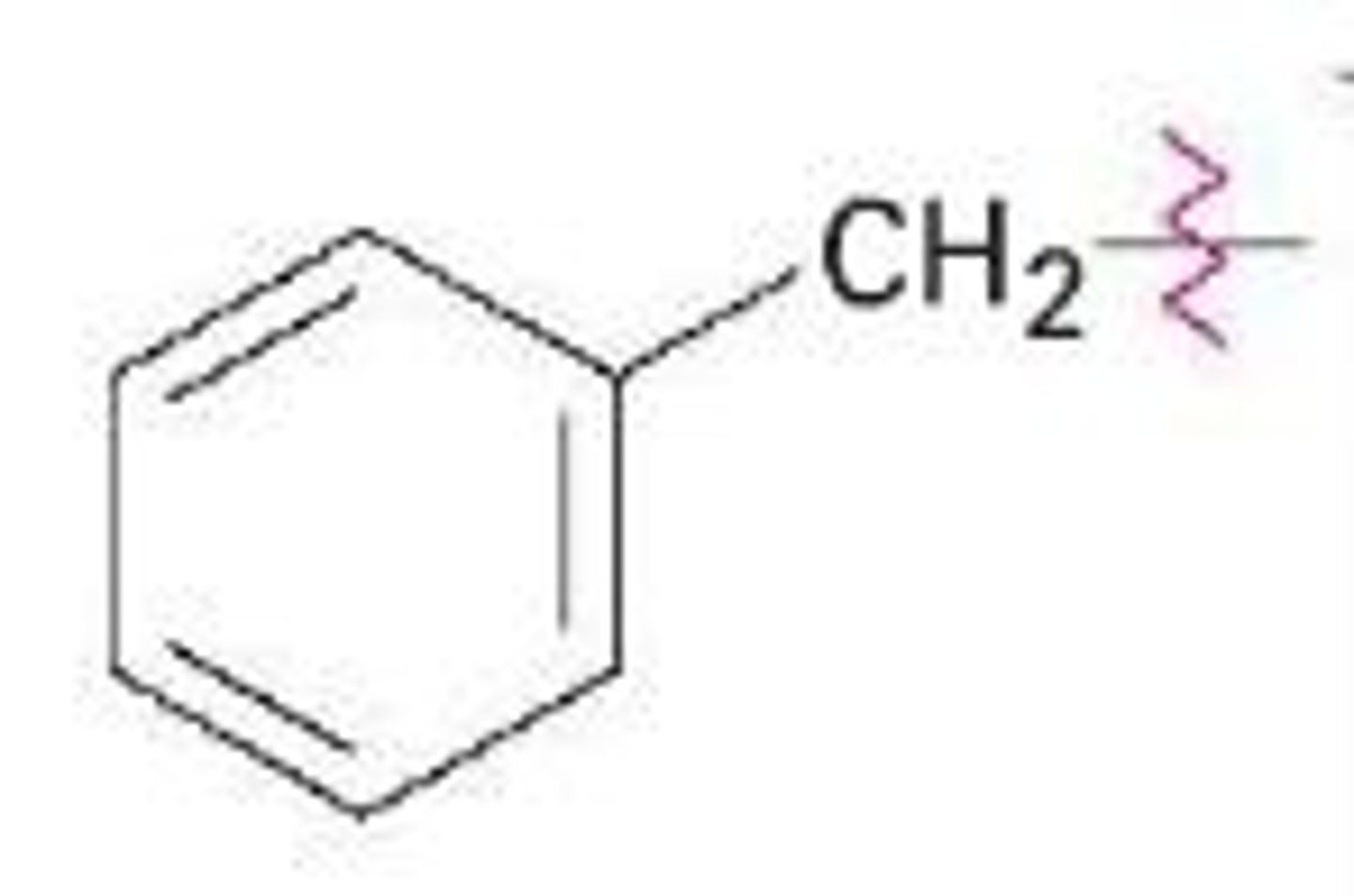
What is benzyl?
A benzene ring with a CH2 group coming off it
When naming aromatics, what name is used for C6H5 (benzene) when it is acting as a substituent?
Phenyl
1,2 substitution =
ortho
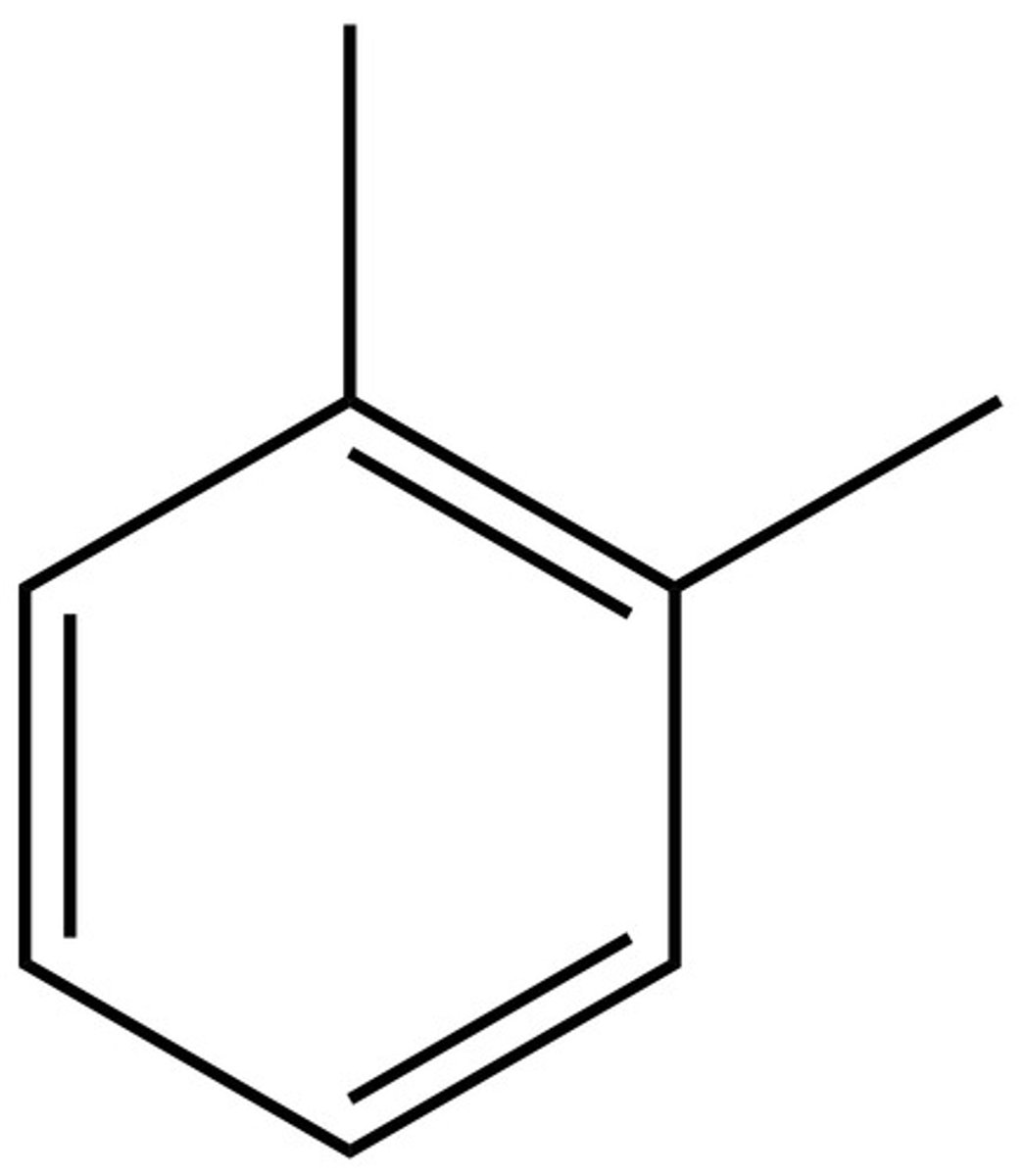
1,3 substitution =
meta
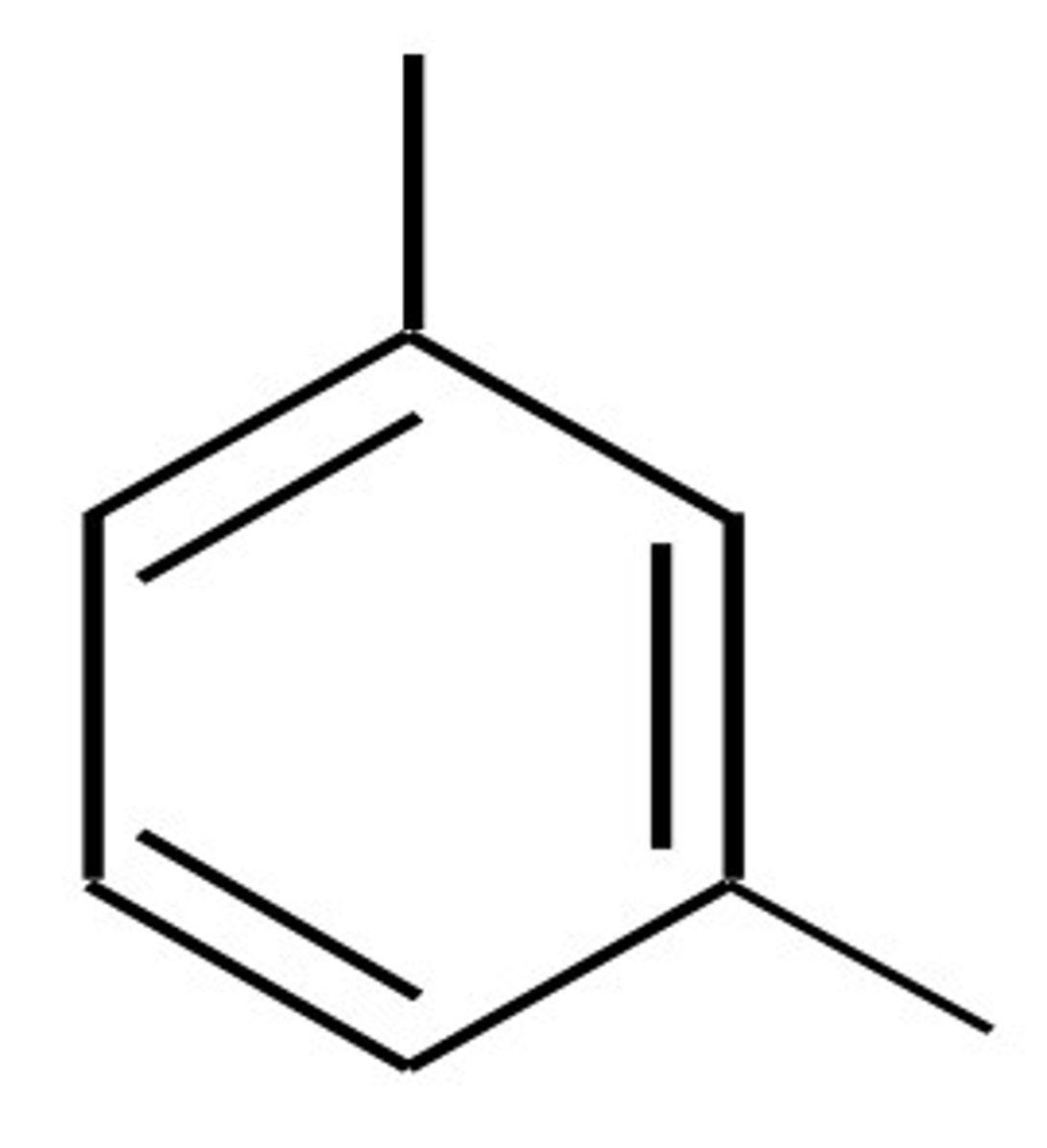
1,4 substitution =
para

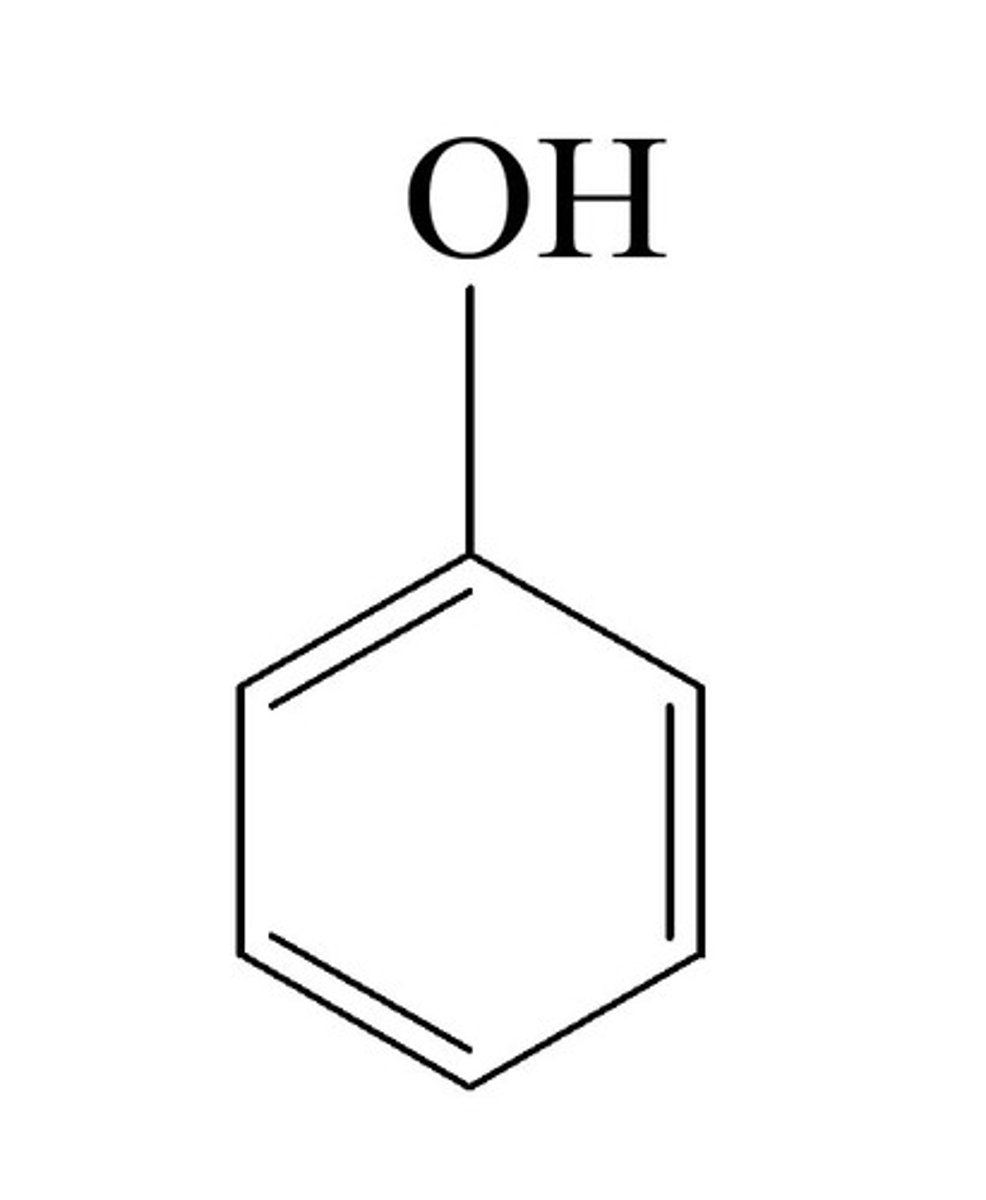
What is phenol?
A benzene ring with 1 OH group
What are the products of an electrophilic aromatic substitution when the reactant is E+?
The H group will be replaced by the E group
What are the products of an aromatic halogenation when the reactant is an X group (Cl, Br, or I)?
The H group will be replaced by an X group (Cl, Br, or I)
What are the products of a chlorination reaction when the products are Cl2 and FeCl3 or AlCl3?
A benzene ring will have a Cl coming off of it
What are the products of a bromonation reaction when the products are Br2and FeCl3 or AlCl3?
A benzene ring will have a Br coming off of it
What are the products of an aromatic reaction when the reactants are I2 and CuCl2?
A benzene ring will have a I coming off of it
What are the products of an aromatic reaction when the products are NO2+ and H2SO4?
A benzene ring will have a NO2 group coming off of it
What are the products of an aromatic reaction when the products are HNO3 and H2SO4?
A benzene ring will have a NO2 group coming off of it
What are the products of an aromatic sulphonylation reaction when the reactants are H2SO4 and heat?
The H on the benzene ring will be replaced with SO3H
What are the products of a friedel-crafts alkylation when the reactants are R-Cl and AlCl3(cat)?
-A benzene ring will have an R group coming off of it
-Possibility for rearrangement of R group
What are the products of a friedel-crafts acylation when the reactants are an R group (with C=O) and AlCl3 (cat)?
-A benzene ring will have an R group coming off of it
-NO rearrangement
Donating groups _____________ reactivity and speed of reaction, and withdrawing groups ______________ reactivity and speed of reaction.
increase, decrease
What type of substitution will electron donating groups have?
1,2 (ortho) or 1,4 (para)
What type of substitution will electron withdrawing groups have?
1,3 (meta)
What are the products of a reaction with an ED group (ex. NH2) when the products are Cl2 and FeCl3?
The Cl will either go on carbon 2 or 4
What are the products of a reaction with an EW group (ex. C=O) when the products are Cl2 and FeCl3?
The Cl will go on carbon 3
What is the exception for EW groups that are halogens regarding substitution?
Halogens will have 1,2 or 1,4 substitutions
What are the products of a reaction when there is an OH group coming off benzene and the reactants are HNO3 and H2SO4?
The products will be the OH group and a NO2 group on either carbon 2 or 4
What are the products of a reaction when there is a Br group coming off benzene and the reactants are CH3CH2Cl and AlCl3?
The products will be the OH group and a CH3CH2 on either carbon 2 or 4
What are the products of a reaction when there is a SO3H group coming off benzene and the reactants are HNO3 and H2SO4?
The products will be the SO3H group and a NO2 on carbon 3
What are the products of a benzylic bromination when the reactants are NBS and hv?
A Br group will add to the carbon that is adjacent to the double bond
What are the traits of a benzylic oxidation reaction?
-It involves a strong (ex. KMnO4) or a weak (ex. PCC) oxidant
-The carbon directly attached to the aromatic will add a compound
-For 1°, a carboxylic acid or aldehyde will form
-For 2°, a ketone will form
What are the products for a reduction of aromatic NO2 if the compound is a benzene ring with NO2 and the reactants are H2, Pd or Sn/HCl?
The product will be a benzene ring with a NH2 group (NO2 becomes NH2)
What are the products of a reduction of aromatic ketones if the compound has a carbonyl group and the reactants are H2, Pd or Zn/HCl?
The carbonyl group goes away