Alcohols and Organic Analysis
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
How are alcohols classified as primary, secondary or tertirary?
Primary- Two (or three if methanol) H atoms bonded to the carbon carrying the OH group
Secondary- One H atom bonded to the carbon carrying the OH group
Tertiary- No H atoms bonded to the carbon carrying the OH group
What conditions are required for the dehydration of alcohols?
Alcohols undergo dehydration reactions through elimination reactions when heated with concentrated sulfuric acid at 450K
Since water is eliminated, we refer to this as a dehydration reaction
What is a dehydration reaction?
A reaction where water is lost from a compound
What is the difference between condensation and dehydration reactions?
Condensation reactions remove water to join molecules
Dehydration reactions just remove water from a compound
What is the equation for the dehydration of ethanol?
CH3CH2OH (g) → CH2=CH2 (g) + H2O (l)
H2SO4 above arrow
Outline and name the mechanism for the dehydration of alcohols?
(Acid-catalysed) elimination
A bond forms as a lone pair of electrons on oxygen forms a bond with a H+ ion
This forms an intermediate where the oxygen is bonded to two hydrogens and has a positive charge
The pair of electrons in the C-O bond move to the positively charged oxygen, breaking the bond
This forms a carbocation intermediate and water
The electrons in one of the C-H bonds in the carbon adjacent to the positively charged carbon moves to the C-C bond forming a C=C bond and regenerating a H+ ion
This forms an alkene and a H+ ion, so the H+ ion acts as a catalyst
What two ways can ethanol be made industrially?
Direct hydration of ethene with steam
Fermentation
What is the equation for the direct hydration of ethene with steam and what is the reaction catalysed by?
CH2=CH2 (g)+ H2O (g) → CH3CH2OH (g)
Catalysed by phosphoric acid (show above arrow)
What conditions are needed for the direct hydration of ethene with steam?
A phosphoric acid catalyst (H3PO4)
570K
6-7 MPa of pressure
Name and outline the mechanism for the hydration of ethene with steam
Electrophilic addition
The H+ ion (an electrophile) is attracted to the dense region of negative space in the C=C bond
The double bond breaks, forming a C-H bond and a carbocation intermediate
Water then reacts with this carbocation to from a C-O bond with two hydrogens bonded to the oxygen, making the oxygen positive
One of the O-H bonds breaks as electrons move to the positive oxygen
This forms an alcohol and a H+ ion, so the H+ ion acts as a catalyst as it is regenerated
Why are high temperatures and pressures used for the direct hydration of ethene with steam?
To prevent the reaction from moving backwards
How does industrial fermentation work?
Sugar cane, molasses or starch is broken down into glucose for fermentation
Zymase in yeast catalyses fermentation
The product is a mixture of water and about 15% ethanol by volume
What conditions are needed for industrial fermentation?
Aqueous glucose
Yeast catalyst and 310K
100 KPa
Anaerobic conditions
What is the equation for fermentation?
C6H12O6 → 2CH3CH2OH + 2CO2
What are the advantages of direct hydration of ethene with steam for the production of ethanol?
Produces more ethanol
Faster
No waste products
100% Atom economy
What are the disadvantages of direct hydration of ethene with steam for the production of ethanol?
High temperature and pressure = money
Ethanol is produced in the gaseous state
Depends on non-renewable resource- ethene
What are the advantages of industrial fermentation for the production of ethanol?
No high temperatures or pressures- less money
Depends on renewable source- yeast
CO2 can be used as a feedstock
What are the disadvantages of industrial fermentation for the production of ethanol?
Only produces 15% ethanol by volume
Waste product of CO2 increases carbon emissions
Temperature has to be kept constant for yeast enzymes
Slower
A lot of natural materials like sugar cane or potato needed
Yeast sensitive to temp, pH
Requires a substantial amount of water
How can primary alcohols be oxidised and what are they oxidised to?
Primary alcohols are oxidised to aldehydes by and oxidising agent called potassium dichromate (VI) which is acidified (K2Cr2O7/H+)
What is oxidation?
Gain of oxygen
Loss of electrons
Loss of hydrogen
What is reduction?
Gain of electrons
Loss of oxygen
Gain of hydrogen
How do we show oxidising agents because chemists are lazy?
[o]
Outline the equation to show the oxidation of ethanol?
Draw out ethanol then + [o)
Draw out ethanal + H2O
What are the conditions for the oxidation of ethanol?
Takes place at room temperature
How are aldehydes separated from a mixture when an alcohol is oxidised?
Immediately by distillation
This is because if we leave the solution to react for too long we start to produce carboxylic acids as the aldehyde undergoes further oxidation
When boiling, we break IMFs
Different boiling points are used to separate substances
Draw out the labelled distillation apparatus
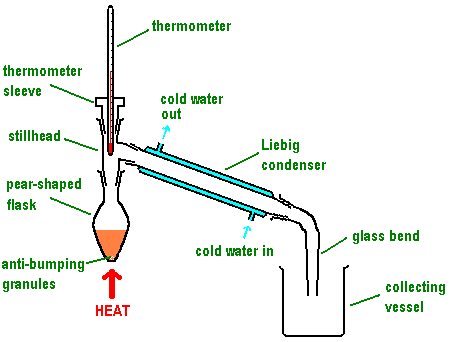
Why does the reaction of alcohol with an oxidising agent have to be distilled immediately?
To prevent further oxidation
What occurs during further reaction of the reaction of an alcohol with an oxidising agent?
Further oxidation of the aldehyde would produce a carboxylic acid
How can we get straight from an alcohol to a carboxylic acid?
Use excess oxidising agent (2x)
Heat under reflux
Draw and label reflux apparatus
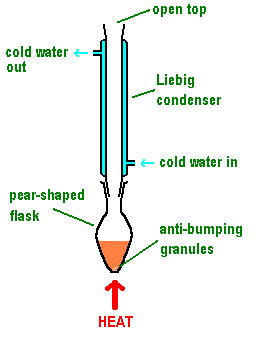
What happens during reflux?
The aldehyde is formed and condensed back into the reaction mixture when vapourised
This forces the aldehyde to be oxidised further
How can primary, secondary and tertiary alcohols be oxidised?
Primary alcohols can be oxidised into aldehydes by acidified potassium dichromate (VI), and then be further oxidised to form carboxylic acids
Secondary alcohols can be oxidised into ketones by acidified potassium dichromate (VI)
Tertiary alcohols cannot be oxidised further as the carbon that carries the OH bond is bonded to no hydrogens
How do we test for alcohols using oxidising agents?
Add aqueous potassium dichromate (VI) acidified by dilute sulfuric acid
Solution turns from orange to green
What is a limitation of a test with alcohols
We cannot identify tertiary alcohols
Why does a solution turn from orange to green when testing for an alcohol?
Cr2O72- is reduced to 2Cr3+
Cr2O72- (dichromate ions) are orange and 2Cr3+ is green
What tests can we use for aldehydes?
Tollen’s reagent
Fehling’s solution
How does Tollen’s reagent identify an aldehyde?
Reagent is ammoniacal silver nitrate [Ag(NH3)2]+NO3-
This acts as a mild oxidising agent
When the aldehyde is oxidised, it forms a carboxylic acid
Silver atoms form a silver mirror
What is the ionic equation to show the formation of a silver mirror
[Ag(NH3)2]+ + e- → Ag(s) + 2NH3 (aq)
How does Fehling’s solution identify an aldehyde?
Is a blue solution containing Copper (II) ions (Cu2+) in an alkaline solution (NaOH)
Cu2+ are mild oxidising agents- aldehydes oxidised to form a carboxylic acid
Aldehydes → Carboxylic acid + e-
Cu2+ ions are blue while Cu+ ions are red, so a brick red precipitate forms as oxidising agents are themselves reduced, gaining electrons
Write the ionic equation for the formation of a brick red precipitate in Fehling’s test
Cu2+(aq) + e- → Cu+(s)
How do we test for alkenes?
Shake alkene with bromine water
Orange solution becomes colourless
What test can we conduct to identify carboxylic acids?
Pour some test solution into a test tube containing sodium carbonate solid
Observe fizzing
What is the word equation that shows what happens when an acid and carbonate react?
Acid + Carbonate → Salt + Carbon Dioxide + Water
What is the equation for the reaction of ethanoic acid with Sodium carbonate?
2CH3COOH + Na2CO3 → 2CH3COONa (sodium ethanoate) + CO2 + H2O
How can we test for halide ions/halogenoalkanes?
Add aqueous sodium hydroxide to a halogenoalkane and warm gently in a water bath- nucleophilic substitution occurs, releasing halide ions
Add aqueous acidified silver nitrate
Chloride ions form a white precipitate
Bromide ions form a cream precipitate
Iodide ions form a yellow precipitate
Why can 2,2-dimethylpropan-1-ol not be dehydrated? (This is just an example)
Because the carbon adjacent to the carbon carrying the OH group has no hydrogens attached
This means that when the carbocation forms, there is no C-H bond on the adjacent carbon that can be broken in order to form a double bond
What is high resolution mass spectroscopy?
We can determine the m/z of a peak to several decimal places
This allows us to distinguish between compounds with similar Mr but different empirical formula
What is infrared spectroscopy?
IR spec allows us to determine what functional groups are within a compound
Any wavelength absorbed by the sample being examined will form a peak in a graph which can be used to determine the functional group
Different bonds absorb IR at different frequencies
What is the wave number of a bond affected by in IR spec?
The bond enthalpy
The masses of the atoms
What is the fingerprint region?
Region below 1500-400 wavenumbers
Allows experienced chemists to determine the exact compound
It is unique in every compound
Sample spectrum is compared with database
What does the peak for O-H in alcohols looks like?
Broad peak
Occurs at 3230-3550 (above 3000)
What is the peak for C-H bonds shaped like?
Split into a few jagged peaks
Occurs at 2850-3300
What does the peak of an O-H bond in carboxylic acids look like?
Less smooth and more broad than O-H in alcohols
Occurs between 2500-3000
There is also a small peak on the side showing a C-H bond
What does a C=O bond look like on a IR spectrum?
Long, pointy and not jagged
Occurs between 1680-1750