Gas Laws AP Chem
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Gases:
uniformly fill any container
mix completely with any other gas
exert pressure on its surroundings
Standard conditions, 1 mol:
22.4 L at 0°C, and 1 atm
Pressure =
force / area
-molecules stricking an area on the wall
SI units
Newton/meter² = 1 Pascal (Pa)
1 standard atmosphere = 101,325 Pa
NOTE: difference between Pa and kPa
1 standard atmosphere = 1 atm = 760 mmHg = 760 torr
NOTE: these are on reference sheet (the important ones)
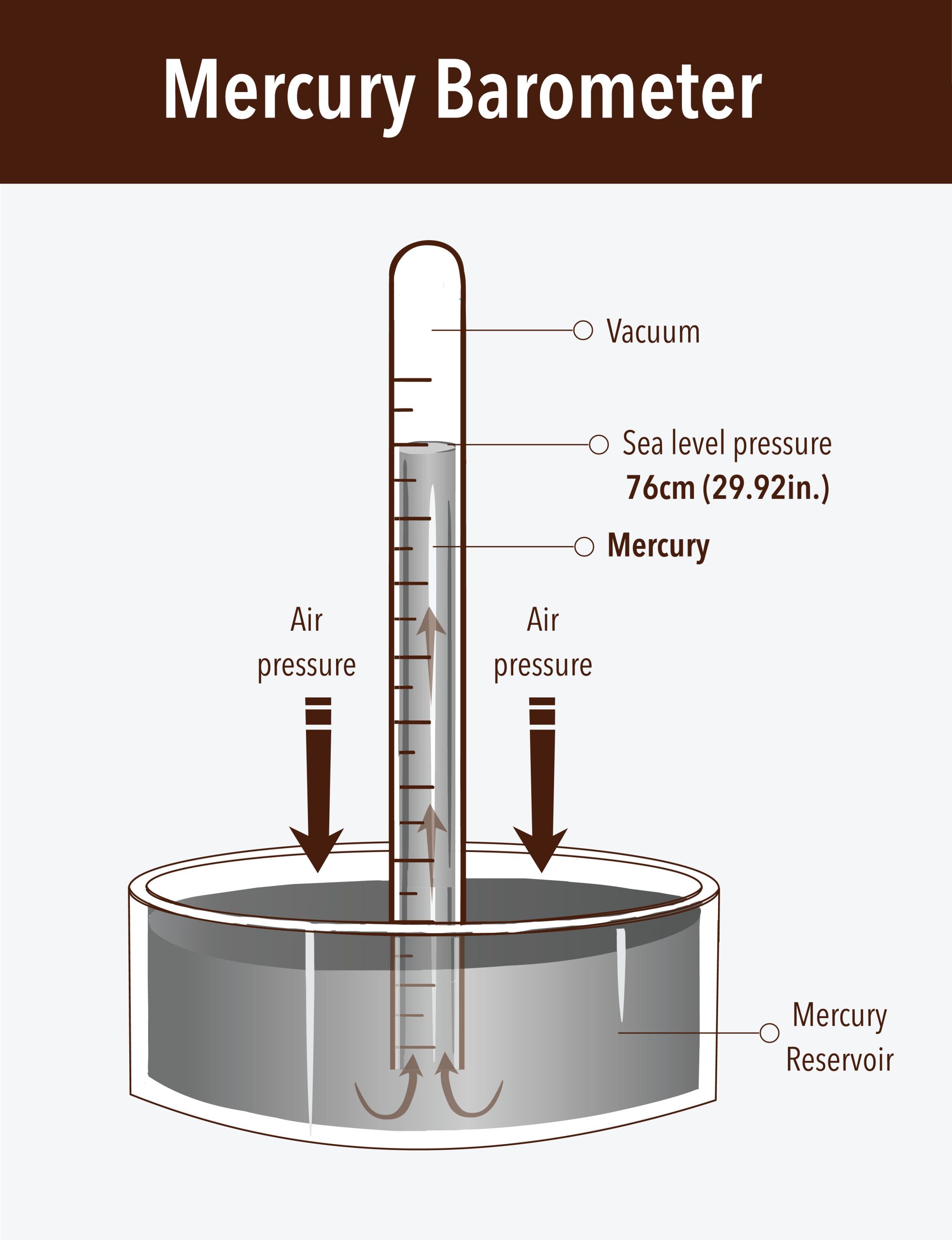
Mercury Barometer
-used to measure atmospheric pressure
-mercury flows out of the tube until the pressure of the column of mercury standing on the surface of the mercury is equal to the pressure of the air of the rest of the surface of mercury
(Honestly just look at the picture to understand)
Manometer
Device used for measuring the pressure of a gas in a container (lowkey unimportant)
Boyles Law
INVERSELY RELATED
Pressure and Volume
P1V1 = P2V2
only linear relationship
other variables MUST be held constant
Charles Law
DIRECT RELATIONSHIP
volume and temperature
V1/T1 = V2/T2
V = bT (b is proportionally constant)
Temperature MUST be in Kelvin
(C + 273.15)
Avogadro’s Law
DIRECT RELATIONSHIP
Moles and Volumes are proportional
n1/v1 = n2/v2
V = an (a is a proportionality constant)
1 mol of any gas at STP = 22.4L
STP = 0C, 1 atm
Combined Gas Law
P1V1/T1 = P2V2/T2
Ideal Gas Law
All together
PV = nRT
R = universal gas constant 0.08206 (acheived by plugging in standard conditions)
Molar volume of an Ideal Gas
1 mol of ideal gas at 0C and 1 atm, volume is 22.4L
Molar Mass of a gas
D = PM/RT
D= density g/L
M= molar mass g/mol
Daltons Law of Partial Pressure
Ptotal = P1 + P2 + P3 + …
-total pressure exerted is the sum of pressures of each gas would exert if it were alone
Non Ideal Gases, Postulate 3
Particles do not attract or repel
→ NOT common
Conditions that make a non-ideal gas
Pressure is high (Volume is low)
Temperature is low
Under these conditions:
-concentration of the gas particles is high
-IMFs can no longer be ignored
low temp. slows the particles down so they’re less likely to not attract or repel
Which gases deviate the least from ideality:
Lightest gases, and non polar gases
Strength order of IMFs:
Hydrogen Bond (H-N, H-O, H-F)
Dipole - Dipole (Polar molecules)
London Dispertion Forces (non polar molecules, more electrons = stronger LDF)
For a real gas actual pressure is …
lower than the pressure of an expected ideal gas
If gas patricles have IMFs…
pressure will be less than expected
The Measure of the speed of particles in a gas equation:
R = 8.3145 J/Kxmol (J = Joule = Kg x m² / s²)
T = temp of gas in K
M = molar mass of a mole of gas IN KILOGRAMS
final units in m/s
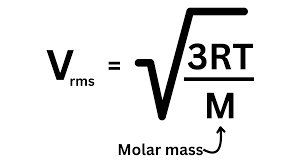
Calculating Average Kinetic Energy of a Gas:
1/2(RT)
R= 8.3145 J/Kmol
T= temp in K
final units in J/mol
rate of effusion equation
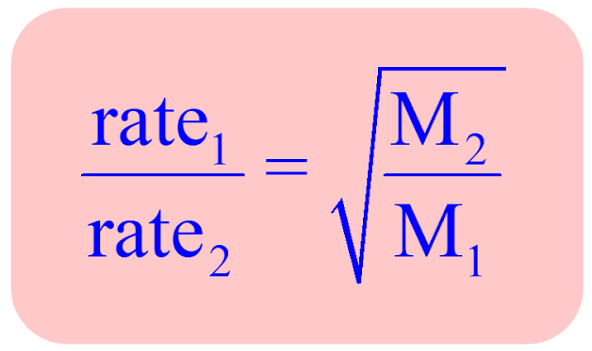
Conditions for ideality:
High temperature and low pressure
Which means there’s no IMFs so the gas molecules are insignificant
If it doesn’t behave ideally…
Pressure increases and volume of gas particles become significant
Pressure affects the volume since…
Now there’s a value in the container, because of the IMFs which clump particles together so now particles combine less
Kinetic Molecular Theory postulates:
1) gas particles are so small they’re negligible so volume of a gas particle can be ignored
2) gas particles are in constant motion, when the particles bump into the walls this results in pressure
3) particles are assumed to not attract or repel therefore IMFs can be ignored for an ideal gas
4) the avg KE is proportional to its kelvin temp: KE = 1/2mv²
Maxwell Boltzmann distribution:
Distribution of the kinetic energies of particles at a given temperature
Higher speed means less molecules sine there’s less collisions
(Spread your legs)
When you increase temperature..
Not every particle has the same KE, temp is the avg of the KE NOT its peak
Avg KE
Temp in Kelvin
1/2mv²
m: KILOGRAMS
KE: Joules
v: m/s
Velocity
Speed, m/s