Lec 5 | Etching |MS 3012
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
A smaller Airy disk is needed so that smaller patterns can be defined
true or false
true
Lithography using which of the following light sources will require better wafer planarization or reduced surface roughness
g-line (Hg source)
i-line (Hg source)
KrF Laser
ArF Laser
ArF Laser
(Depth of Focus is the ability of lensing system to project img on top of a surface. So, if u have a rough surface, the image won’t be sharp at every plane. So, u want a large DOF. If the wafer needs a smooth, u have a small DOF. DOF is small when u have a small wavelength and ArF has the smallest wavelength. Depth of Focus: The depth of focus in lithography decreases with shorter wavelengths. A reduced depth of focus means the process is less tolerant to variations in wafer surface height. Therefore, better wafer planarization (smoother surfaces) is needed to keep the entire wafer surface within the focus range and ensure precise patterning.)
T/F In wet etching, the conditions are controlled such that the etchant transport is the slowest process
F
The role of HNO3 in a HNO3:H2O:HF:CH3COOH etchant solution for Si etching is as a…
Oxidizing agent
Essentially, in the context of etching, what is selectivity?
How well can the solution attack the thin film you want it to attack while leaving everything else alone
What is etching, and what is its primary purpose in micro/nano-fabrication?
Etching is the selective removal of deposited thin-film material to leave a desired pattern on the wafer surface. It is used to define structures and features during micro/nano-fabrication. (Slide 8)
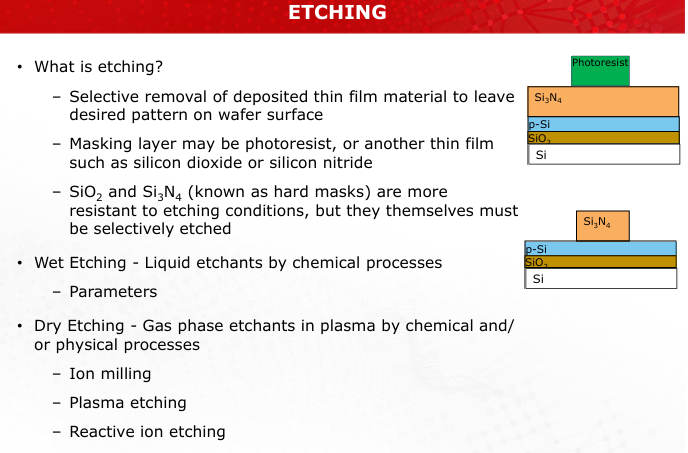
Differentiate between wet etching and dry etching. Include examples of materials used in masking.
Wet Etching: Uses liquid etchants, typically isotropic, and is chemically controlled. Example: Buffered HF for SiO2 etching.
Dry Etching: Uses plasma or ion bombardment, can be anisotropic or isotropic. Example: Reactive Ion Etching (RIE).
Mask materials include photoresist, SiO2, or Si3N4. (Slide 8)
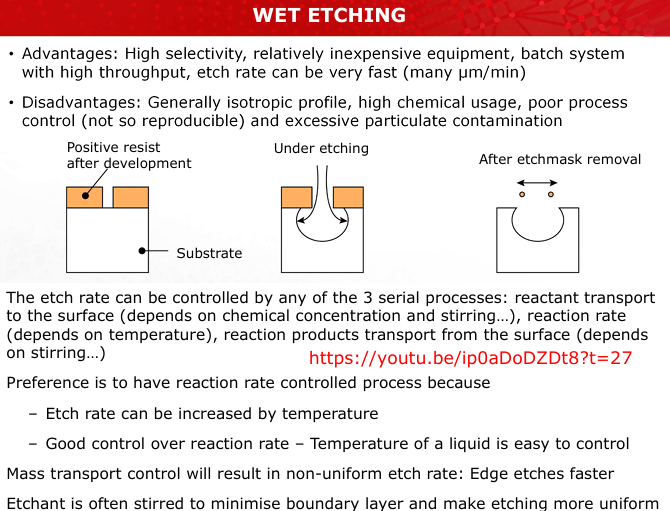
What are the advantages and disadvantages of wet etching?
Advantages: High selectivity, cost-effective, batch processing, fast etch rates.
Disadvantages: Generally isotropic, poor process control, high chemical usage, contamination risk. (Slide 14)
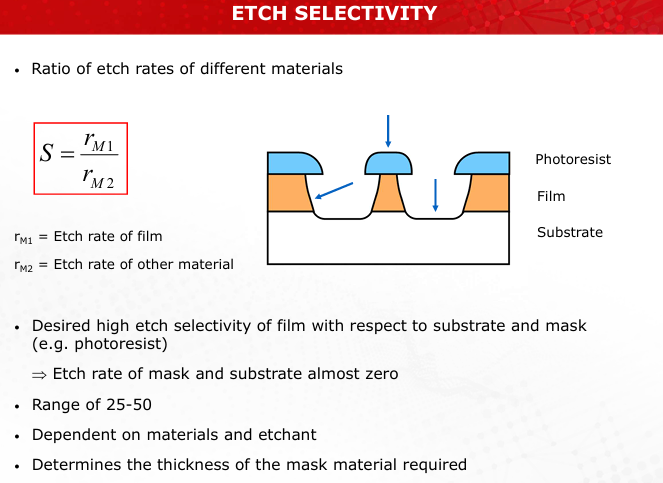
Explain the principle behind the selectivity of etchants. How does this influence the process outcome?
Selectivity is the ratio of etch rates of the target film to other materials like the substrate or mask. High selectivity ensures minimal damage to non-target materials, enabling precise patterning. (Slide 11)
Describe the three mechanisms controlling wet etching rates. Which mechanism offers the most control, and why?
Reactant transport to the surface: Depends on stirring and concentration.
Reaction rate: Temperature-dependent, most controllable.
Product transport away from the surface: Depends on stirring. Reaction rate control is preferred because temperature is easily managed. (Slide 14)
Define etch selectivity and its mathematical representation. Why is high selectivity important?
Selectivity (S) = Etch rate of film / Etch rate of other material. High selectivity ensures that the mask and substrate are minimally affected while targeting the desired layer. (Slide 11)
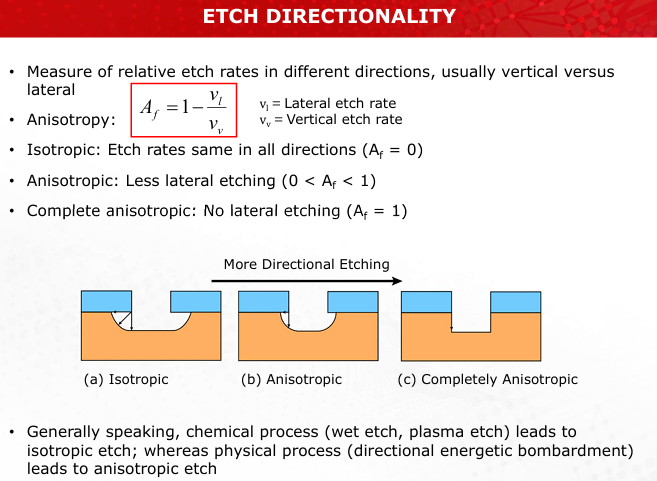
Compare isotropic and anisotropic etching. How does anisotropy affect feature precision?
Isotropic: Equal etch rates in all directions, leading to undercutting.
Anisotropic: Higher vertical etching, reducing lateral erosion, ensures finer and more precise features. (Slide 12)
Describe the factors that influence anisotropy in etching. Why is directional etching preferred for high packing density?
Directional etching relies on ion bombardment for vertical etching, preferred because it maintains the integrity of small and densely packed features. (Slide 12)
Discuss the challenges faced in maintaining selectivity during sputter etching
Sputter etching has poor selectivity because ion bombardment affects all materials similarly, causing undesired erosion of the mask or substrate. (Slide 22)
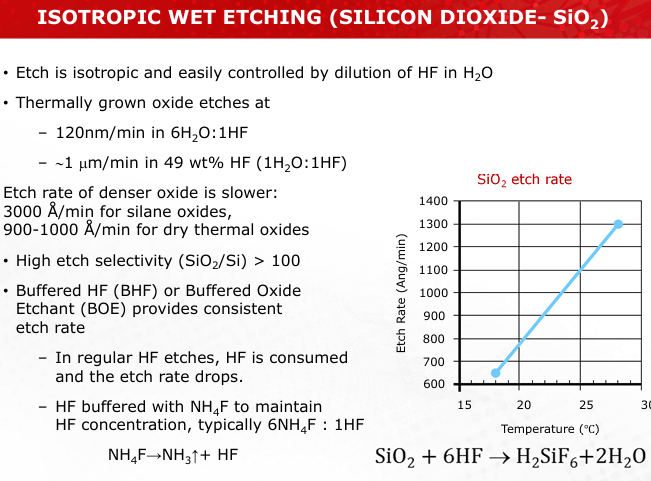
Explain the chemical mechanism of SiO2 etching using HF and BHF. Why is buffering necessary?
HF reacts with SiO2 to form SiF4, which is volatile and removes the oxide layer. Buffering with NH4F stabilizes HF concentration for consistent etching rates. (Slide 15)
How does the composition of HF-HNO3-CH3COOH mixtures affect the isotropic wet etching of silicon? What role does each component play?
HF: Dissolves SiO2 formed during etching.
HNO3: Oxidizes silicon to SiO2.
CH3COOH: Diluent, stabilizes HNO3 to prevent excessive dissociation. (Slide 16)
Discuss the doping-level-dependent etch rates of Si and their applications in etch-stop layers.
Heavily doped Si etches faster in HF-HNO3 mixtures, while lightly doped Si resists EDP etching. This property is used to create etch stops for precise layer definition. (Slide 17)
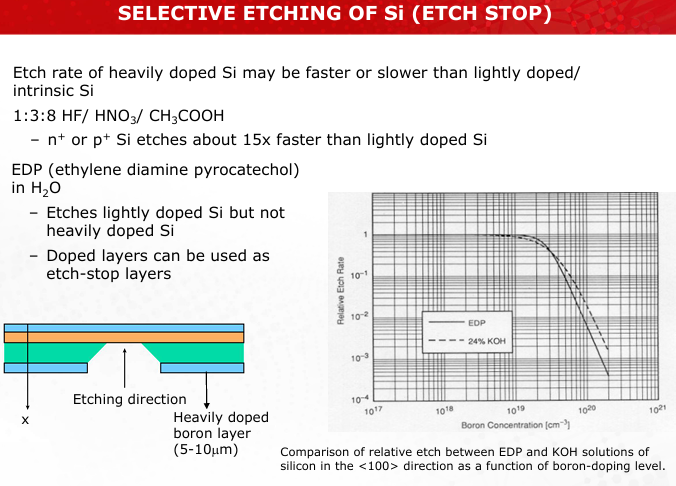
Describe how KOH etching exploits silicon crystallographic effects to create V-grooves or pyramidal pits.
The etch rate is slowest for {111} planes due to their close packing, leading to well-defined angles and shapes, such as V-grooves at 54.7°. (Slides 18-19)
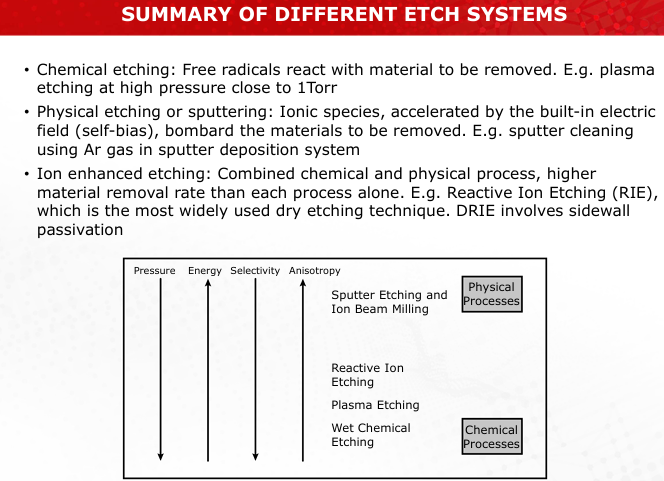
Contrast the mechanisms of sputter etching and plasma etching. Which process is more anisotropic, and why?
Sputter Etching: Physical bombardment, highly anisotropic but non-selective.
Plasma Etching: Chemical reactions, isotropic but selective. (Slides 21, 24)
Explain the role of ions and free radicals in reactive ion etching (RIE). How do they synergistically improve etch quality?
Ions enhance chemical reactions by breaking bonds, while free radicals drive etching. Together, they improve anisotropy and selectivity. (Slide 31)
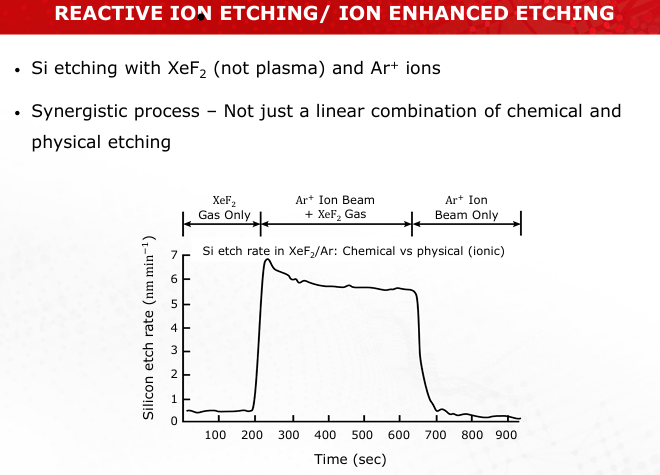
Discuss the differences in selectivity and anisotropy among sputter etching, plasma etching, and RIE.
Sputter: Anisotropic, low selectivity.
Plasma: Isotropic, high selectivity.
RIE: Balances both, offering high precision. (Slides 35-36)
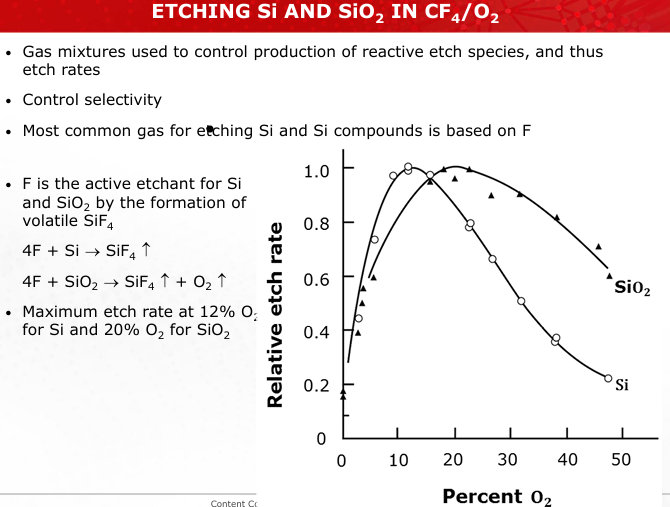
How do gas additives like O2 or H2 in CF4 plasmas influence the etch rates of Si and SiO2? Include chemical equations.
O2: Controls SiF4 formation, improving selectivity.
SiO2+4F→SiF4+O2H2: Reduces F availability, altering etch behavior.
F+H2→HF (slides 27-28)
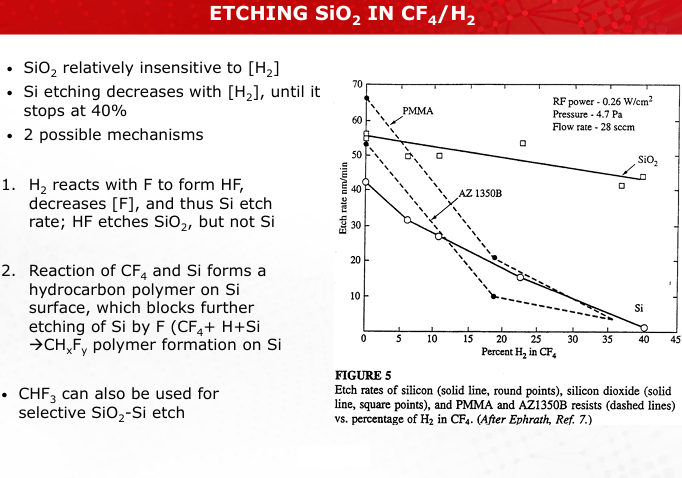
Describe the process and purpose of sidewall passivation in Deep Reactive Ion Etching (DRIE).
Sidewall passivation protects vertical features by depositing a polymer layer, allowing controlled anisotropic etching. (Slide 33)
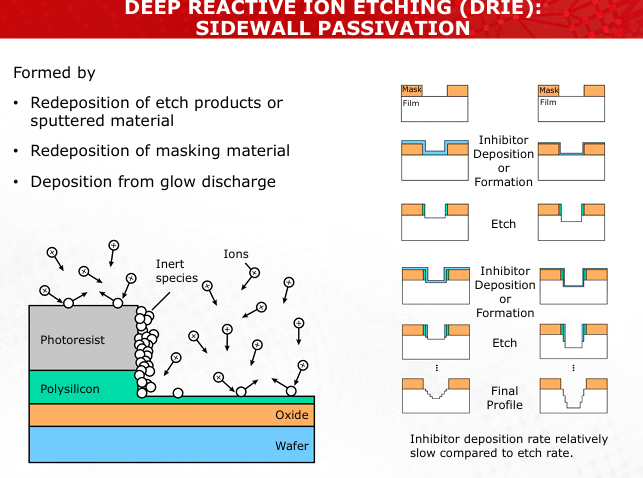
Explain how ion bombardment enhances chemical etching during RIE. Include examples of volatile byproducts formed.
Ions break surface bonds and remove inhibitors, enabling chemical reactions like Si + 4F → SiF4 (Slide 32)
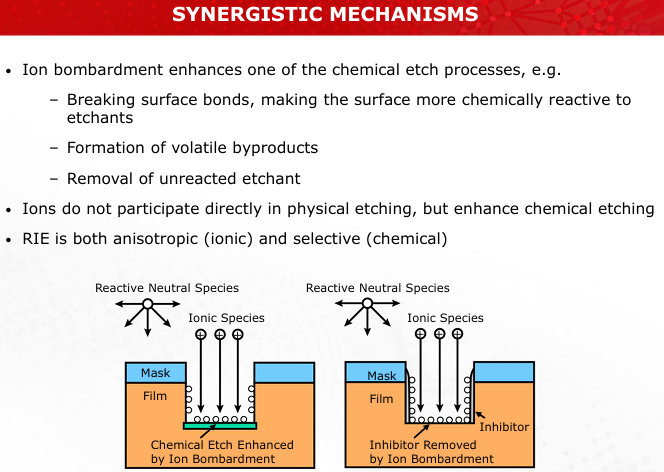
Discuss the trade-offs between high selectivity and anisotropy in dry etching systems. Provide examples of practical applications.
Achieving both can be challenging; RIE balances these for applications like fine semiconductor features. (Slide 35)
Draw and label the etch profiles of isotropic, anisotropic, and completely anisotropic etching
Refer to Slide 12 for clear diagrams.
Sketch the mechanism of wet isotropic etching on SiO2, showing the effect of buffer solutions.
Refer to Slide 15 for reaction flow.
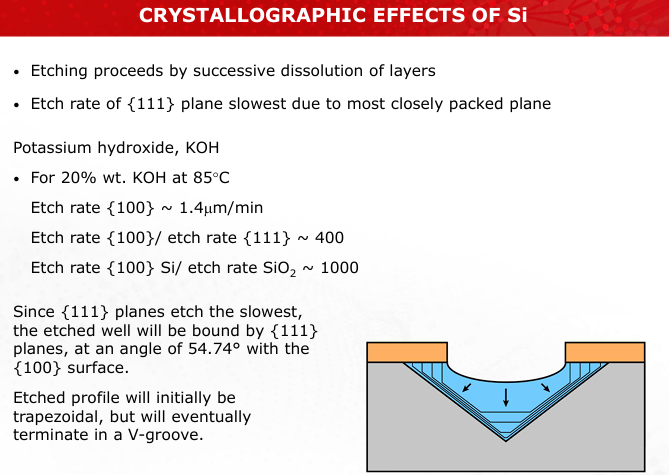
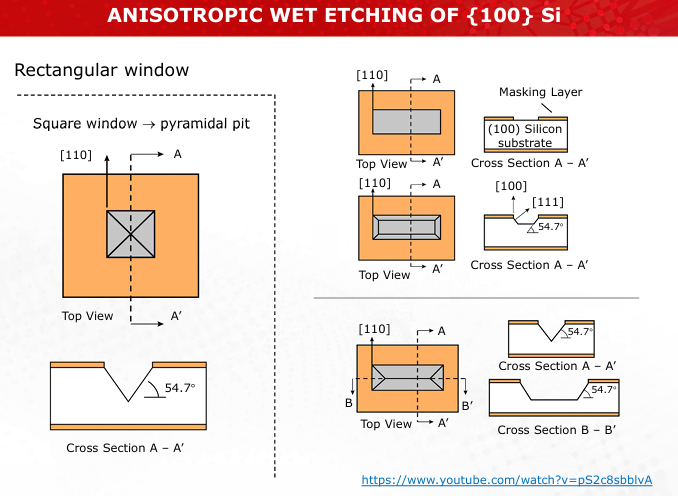
Illustrate the process of KOH-based anisotropic etching, emphasizing the crystallographic orientation of silicon planes.
Refer to Slides 18-19 for etched profiles.
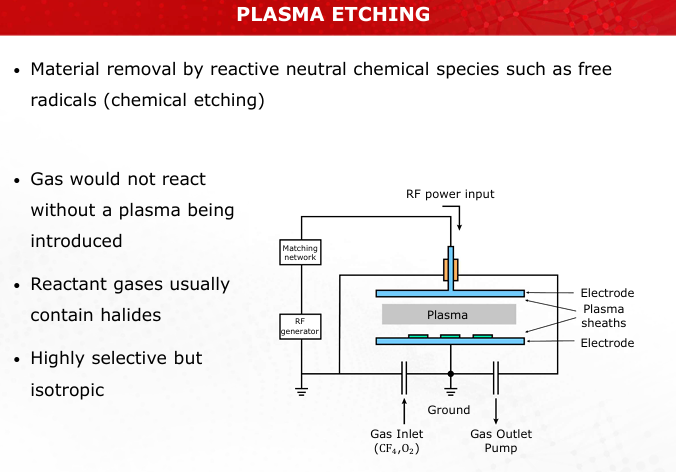
Create a schematic of a plasma etching setup, highlighting the role of RF generators and reactive species.
Refer to Slide 24 for the schematic.