Gas laws, rate of reaction, equilibrium
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
Theoretical ideal gas model
All gases respond the same way to changes in volume, pressure, and temperature when mass of gas is fixed
1)Particles in a gas have negligible volume compared with the volume the gas occupies
2)There are no intermolecular forces between particles, except when the molecules collide
3)Gas particles have a range of speeds and move randomly. Average kinetic energy of particles is proportional to temperature
4) Collisions of particles with walls of the container and each other are elastic, kinetic energy is conserved
Elasticity
When a gas particle collides with the wall of a container, it bounces back with the same speed
Pressure of a gas is result of
A large number of collisions, and is the same on all walls (particles move randomly, no preferred direction)
Volume of a gas is
The total volume of its container as particle spread out to fill all available space
Effect of volume on pressure
volume increases, particles collide less frequently with walls as they take up more space, less pressure
volume decreases, take up less space and collide more frequently with walls, more pressure
Temperature effect on pressure
When temperature increases, particles have more kinetic energy, so collisions with the walls are more frequent and more energetic (particles moving faster), both increasing pressure
When temperature decreases, less kinetic energy, less frequent and less energetic collisions with the walls, less pressure
Deviations of real gases from ideal gas theory
Volume of gases not negligible, actually travel less distance between collisions with the wall, collisions more frequent, pressure is greater than predicted
Attractive forces between particles, when a particle approaches a wall attractive forces from other particles pull in the opposite direction, less energetic collisions and less pressure than predicted
This reduction of speed is most significant when average speed is low at low temperatures
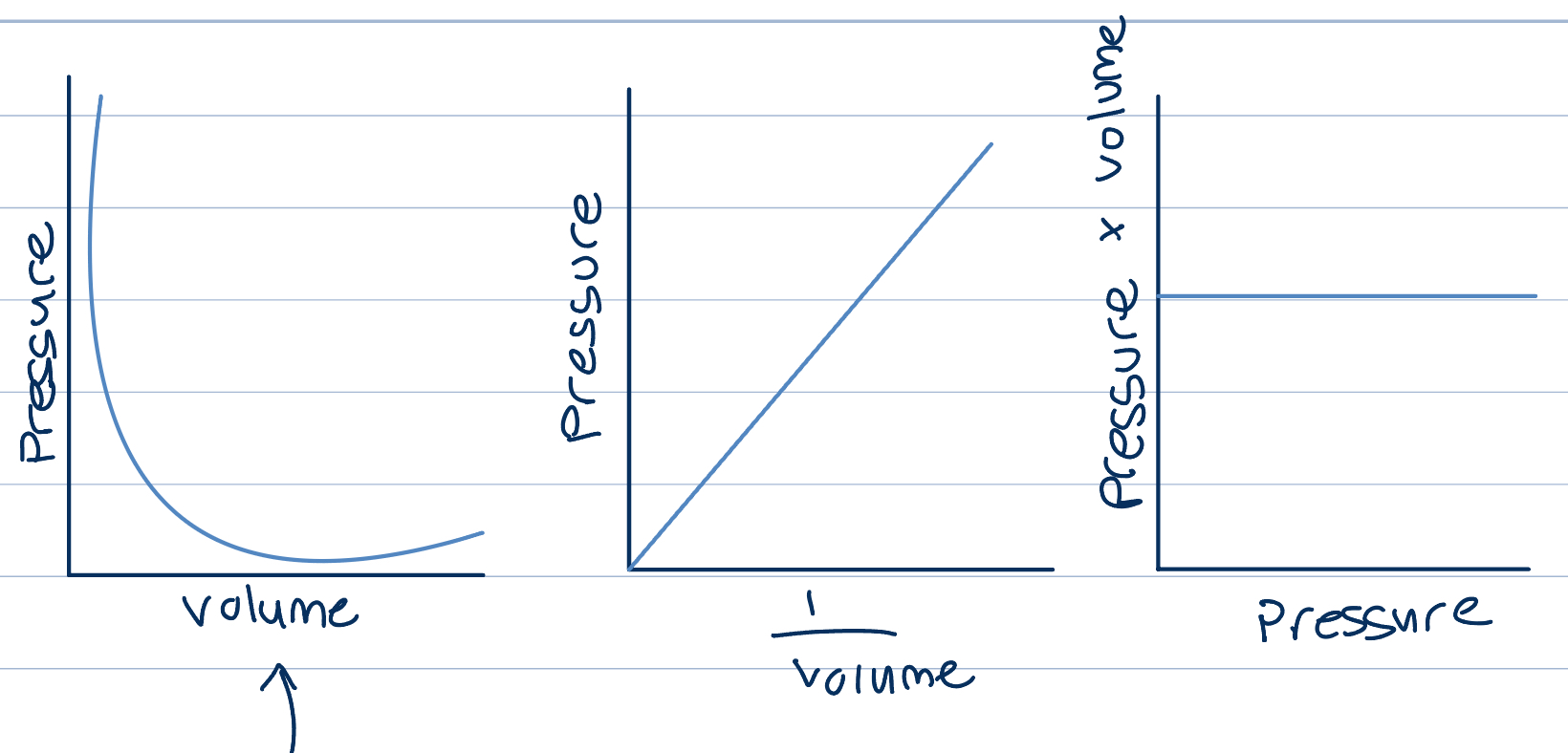
Relation between pressure and volume
If temperature of a gas is held constant, it is found that increasing the pressure of a fixed mass of gas, decreases its volume
Pressure of a gas is inversely proportional to its volume
Product of pressure and volume is a constant
Unit for volume is pascal, Pa equivalent to one n/m² to get from m to cm, divide by 1000 twice, once for dm
Relationship between volume and temperature
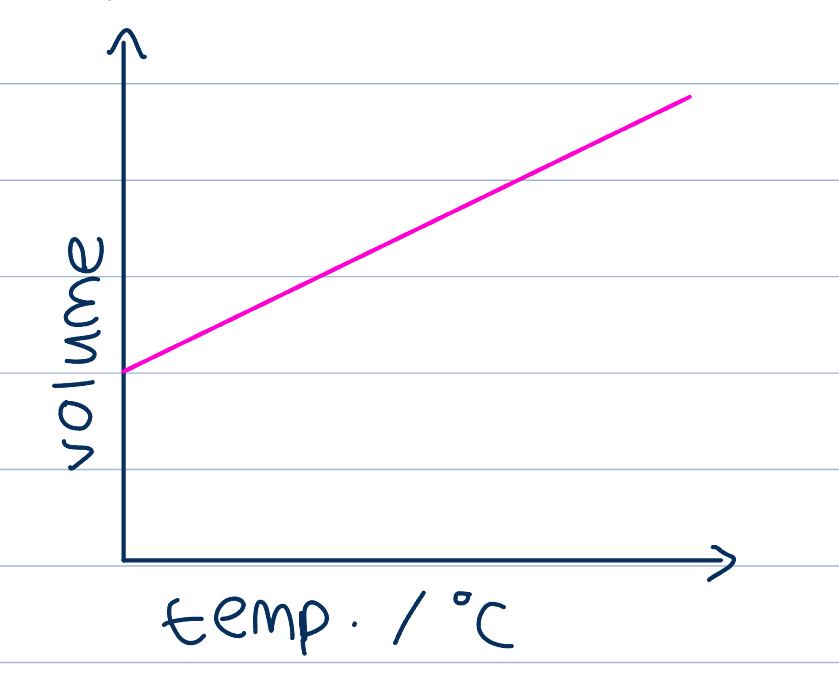
If pressure is kept constant, and temperature is increased, volume will increase
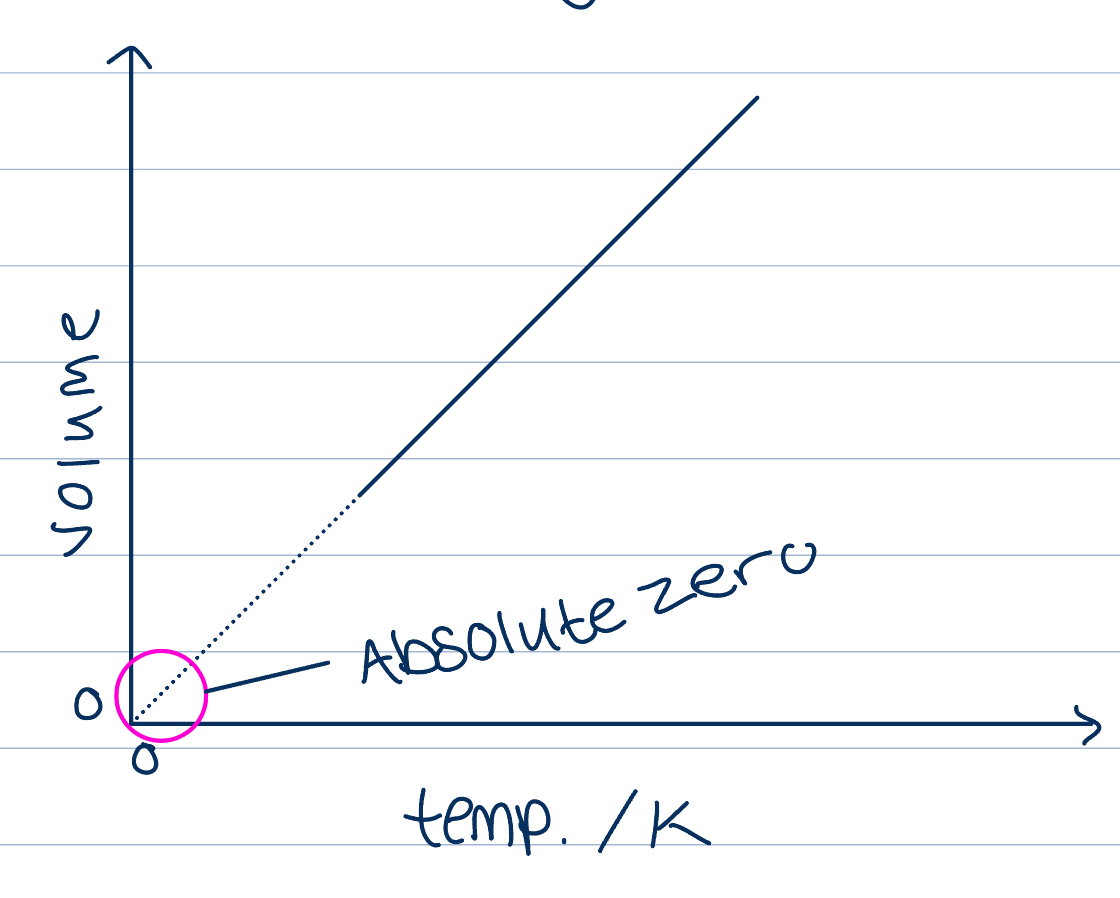
If temperature is measured in degrees Celsius, a linear relationship
When the line is extended backwards, volume is only at zero at -273 degrees, minimum possible temp, volume can’t go below zero
If we use kelvin, relationship is directly proportional, absolute zero is when there is zero volume and that is the minimum temperature
Temperature increase then kinetic energy of particles increase so when particles collide more frequent and with more energy, to keep pressure constant, volume must increase to provide more space and less frequent collisions to keep pressure constant
Units of temperature
Used in Kelvin, absolute temperature, 0K is point when gas particles have no kinetic energy
To get from degrees to kelvin, add 273
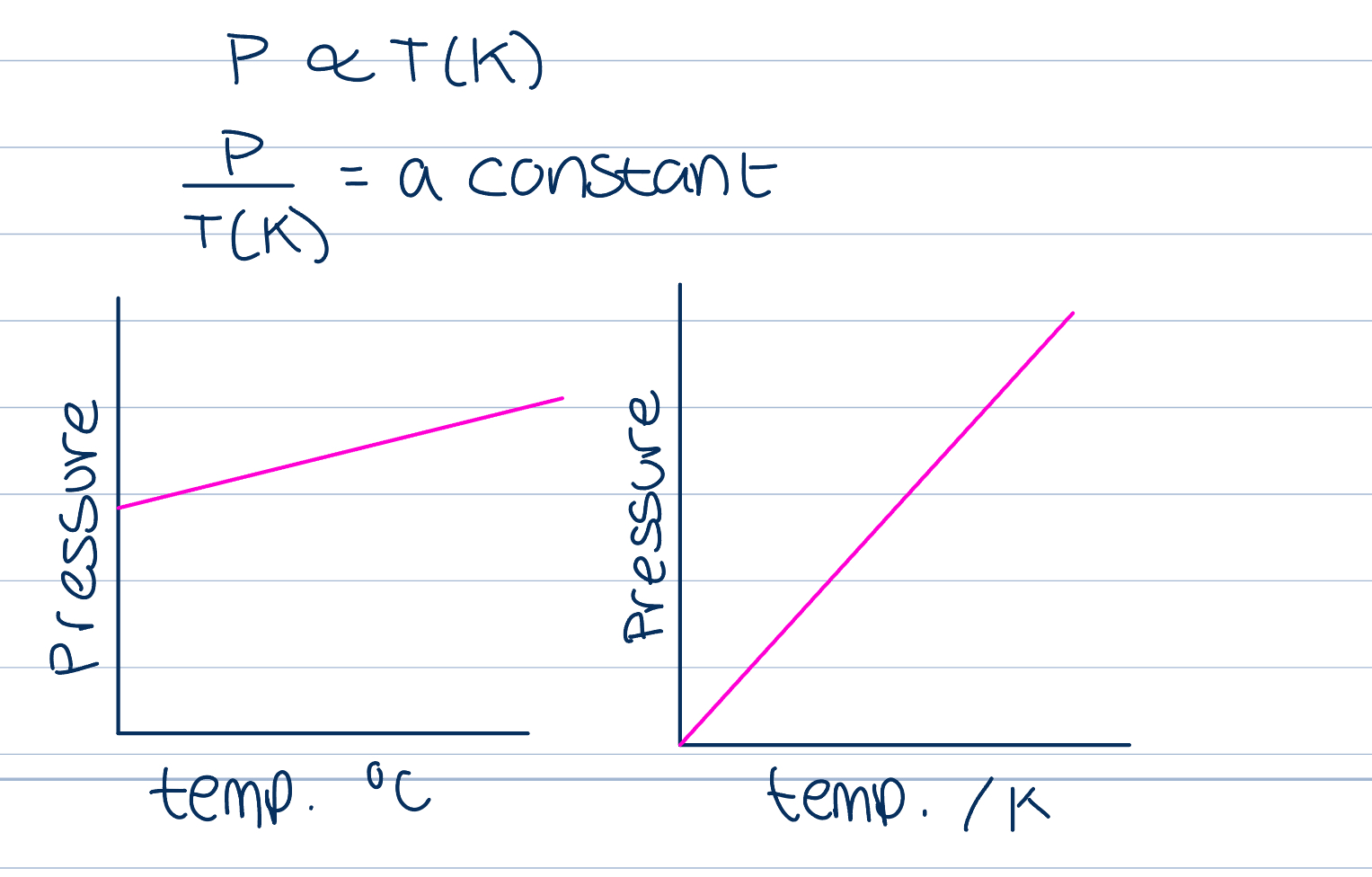
Relationship between pressure and temperature
If volume is held constant, increasing the temperature will increase the pressure, if temperature measured in Kelvin, proportional, otherwise, linear
Increase in temperature increases the average kinetic energy of the particles, causing them to collide more frequently and with more energy with the walls, causing an increase in pressure
Molar volume
Volume occupied by one mole of any gas must be the same for all gases when under STP mv= 22.7 dm³/mol v/mv=n
3 laws for fixed mass of gas
Pressure inversely proportional to volume if temp constant
Temperature proportional to pressure if volume constant and measured in K
Temperature proportional to volume if measured in K and pressure constant
Combined gas law
P1 V1/ T1 = P2 V2/ T2
Cancel out anything it says that stays constant and do not include it
R
universal gas constant
8.31 Nm/K/mol
does not depend on the identity of the gas
Ideal gas equation
PV= NRT
Pressure in Pa (n/m²).
Volume in m³
Number of moles, mass/molar mass
Temperature in K, add 273.15 for if celsius is needed
Density can be found by
mass/volume
Gases deviate most at
Low temperature and high pressure
At low temperature gas particles are closer together have less kinetic energy, more attraction, more like a liquid
High pressure, less volume, closer together, more attraction between particles, more like a liquid
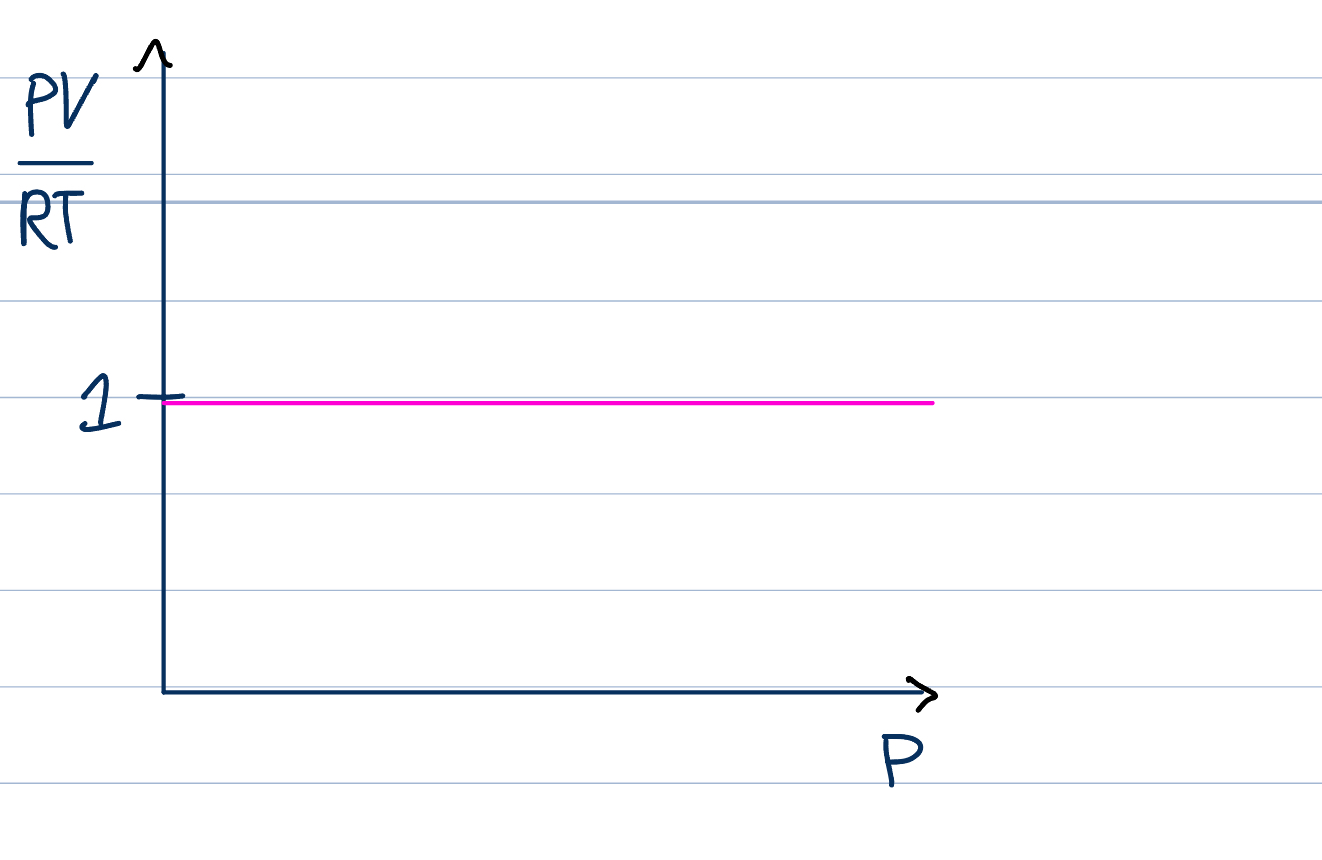
Real gases do not
obey the ideal gas law pv=nrt under all conditions
For one mole of a gas, pv/rt should be equal to one, so graph of pv/rt against p for an ideal gas is a horizontal line with intercept 1
(because when you increase P on one axis, you increase P on the other by the same, so it stays constant)
But, for real gases pv does not equal nrt so the value of pv/rt for one mole will vary
Gases behave most like ideal gas at low pressure and high temperature
Behave least at low temperature and high pressure
If the volume of the gas particles is not negligible, PV/nRT is greater than one, the collisions with the wall are more frequent and pressure is greater, so it will be greater than one
There are attractive forces between the particles, PV/RT > 1, reduces the speed of colliding particles, pressure lower than ideal gas laws, so PV/nRT <1
Rate of a chemical reaction
Speed at which reactants are converted into products during a chemical process
Rate of change in concentration over time
rate= 1/time, 1/s
Concentration rate and instantaneous rate
As the reaction proceeds, reactants converted to products so concentration of reactants decreases and concentration of products increases
ROR= Increase in product concentration over time OR decrease in reactant concentration over time
cannot be negative
Gradient here (rise/ run) is the rate of reaction
Gradient not linear, greatest at start (rate) as this is when the reactant concentration is highest, use as comparison point with different reactions, slows down next
Because of curved gradient, draw tangent to find instantaneous rate and find the gradient of that by making two points
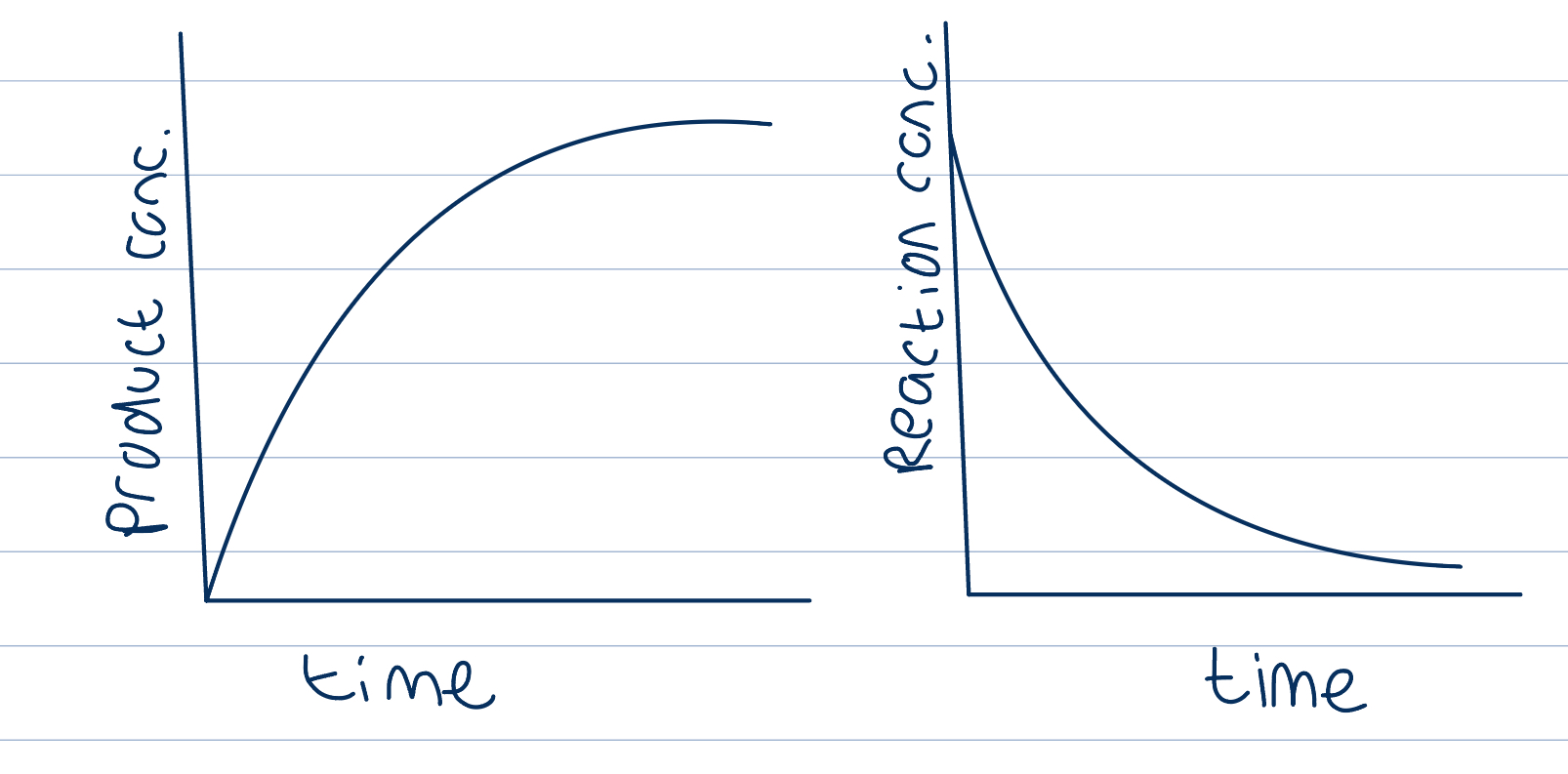
Measuring rates of reaction using different techniques depending on the reaction
What is most convenient for that reaction
Concentration usually not measured directly, but by means of a signal that is relates to changing concentration
Change in gas volume produced
Collect gas and measure change in volume at regular time intervals, allows volume-time graph to be plotted
Gas syringe collects gas released and uses displacement to plot rate
Only if gas collected has low solubility in water
Change in mass
If the reaction is giving off a gas, the corresponding decrease in mass can be measured
Standing the reaction mixture directly on a balance. Method doesn’t work well if hydrogen gas is emitted as it is too light to show significant changes
Allows for continuous readings and a graph to be plotted
Calorimetry/spectrophotometry
Can be used if any reactants or products are coloured
Gives characteristic absorption in the visible region (320-800nm)
Indicator can be added to generate a coloured compound that can then be followed in the reaction
Pass light of specific wavelength through solution and measure intensity of light transmitted by reaction components
Concentration of coloured compound increases so absorbs more light, less light transmitted
Electric current generated according to amount of light transmitted which is connected to a graph of absorbance against time
Decrease in absorbance as coloured reactant is depleted
If it goes colour to colourless, the absorbance decreases until almost all light is transmitted through
Change in concentration measured using titration
Quenching is used, substance is introduced which stops the reaction in the sample at the moment when it is withdrawn
Freeze shot of reaction at an interval time
Repeat this many times at different intervals to see how concentration changes as reaction proceeds
Using conductivity
Total electron conductivity of solution depends on concentration of ions and their charges
If this changes when reactants go to products, Used to follow progress fo reactions
Use a conductivity meter
Machine can be calibrates using solutions of known concentrations so readings can be converted to concentrations of ions present
if 12 ions in reactants and 0 in products, show a decrease in electrical conductivity of the solution as reaction proceeds
Non continuous methods of detecting change during a reaction
Measure time taken to reach a chosen point fixed. Something observable used as an arbitrary end point that signifies to stop the clock
Different reactions take different times and this can be compared
Time is now dependent variable
Only gives an average rate over the time period
Ex time taken for a certain size of magnesium ribbon to react completely with HCl to where it is no longer visible
Magnesium ribbon is independent variable
If you see gas produced or ions in reaction
Use conductivity, gas produced, or mass decrease as rate of reaction measurements
Kinetic molecular theory
Particles in a substance move randomly as a result of their kinetic energy
Because of the random movements and collisions, not all particles in a substance at any one time have the same kinetic energy
Range of values, must take the average, directly related to its absolute temperature (K)
Solid to liquid to gas, increasing average kinetic energy and increasing temperature
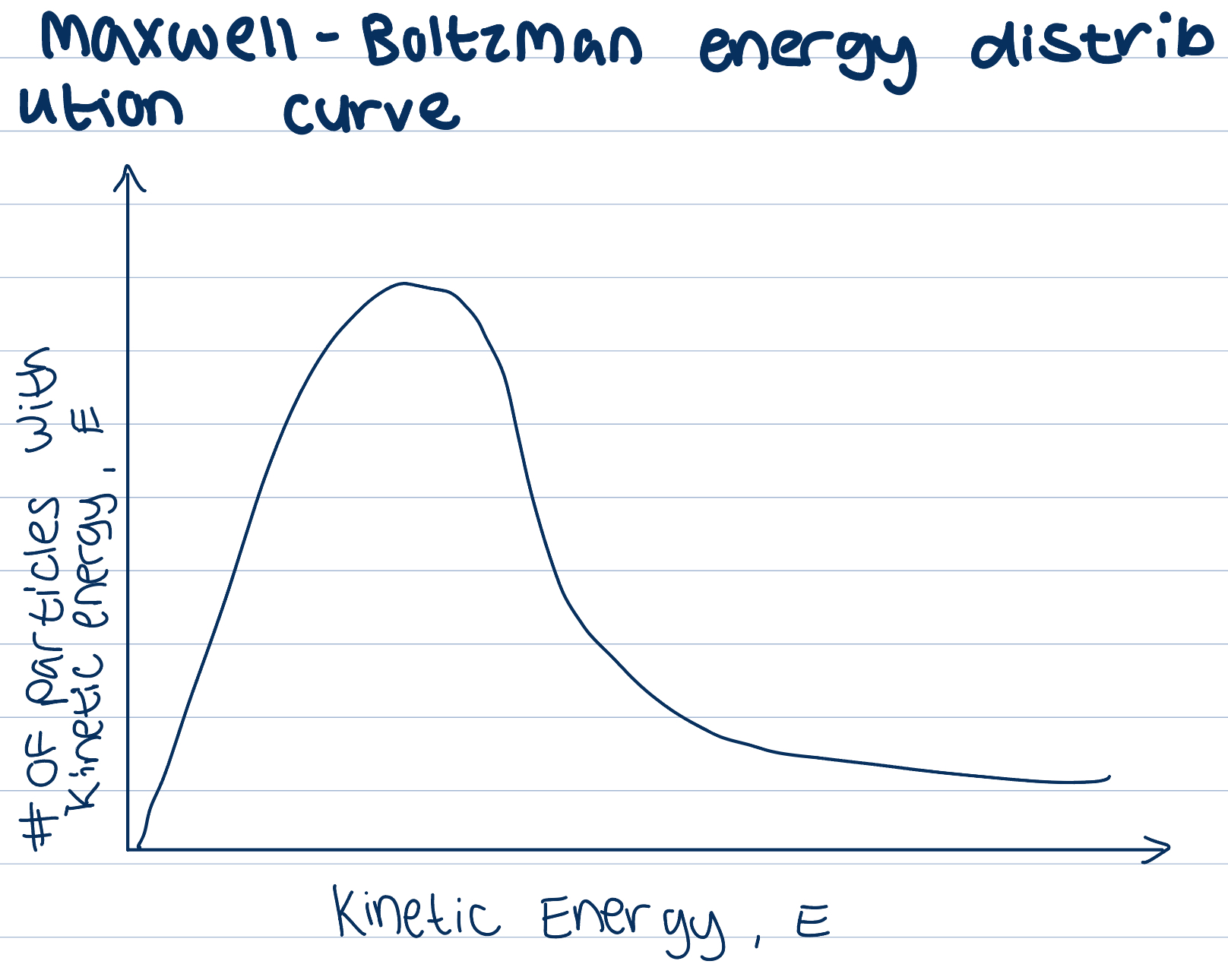
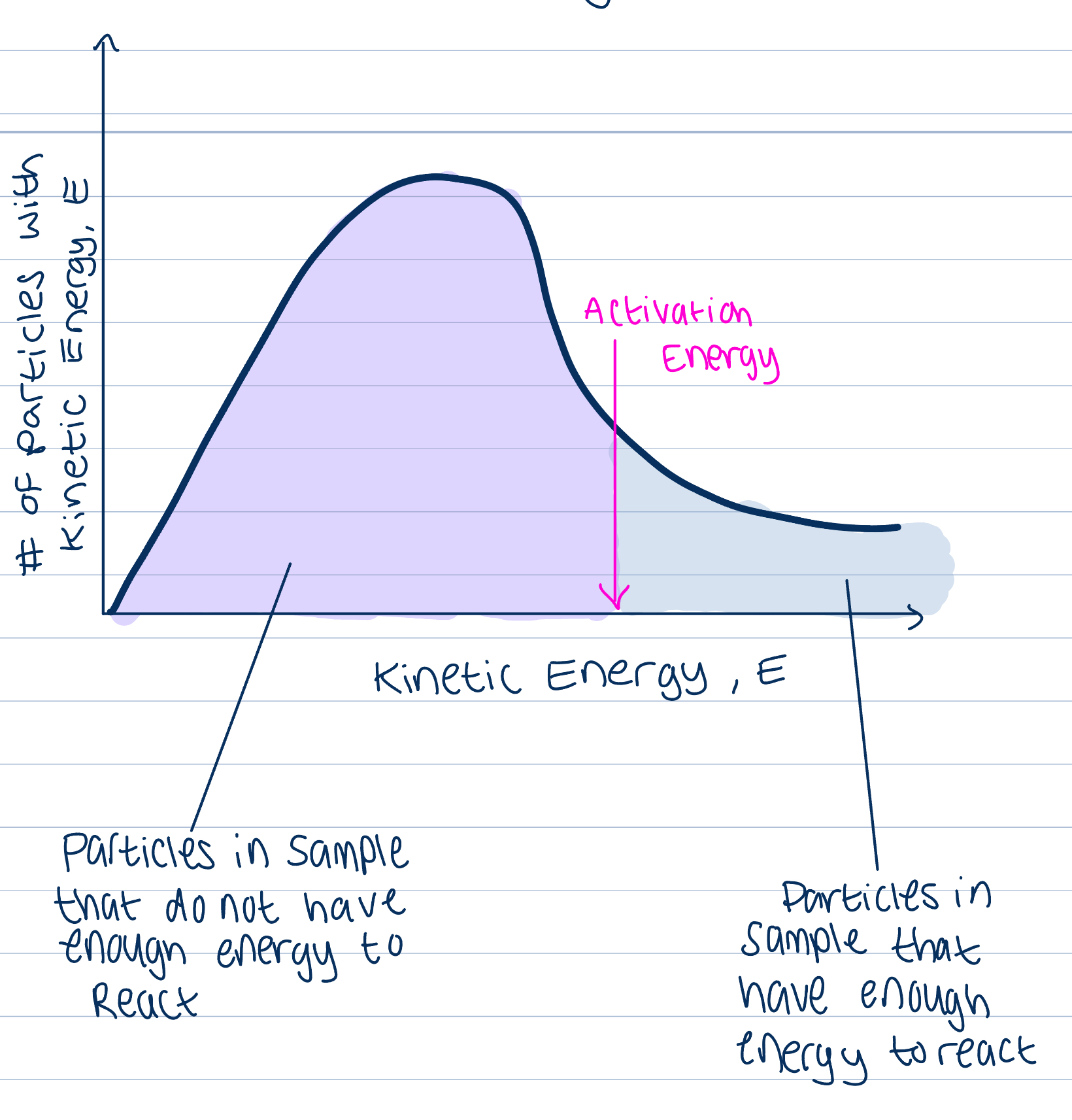
Maxwell Boltzman energy distribution curve
Gas particles at a set temperature show a range in their values of kinetic energy
Area under the curve shows total number of particles in the sample
How reactants form products
Reactants placed together, Kinetic energy of particles causes collisions
Energy of collisions can cause bonds within reactants being broken, and new bonds forming
Resulting in products forming and reaction happening
rate of reaction depends on number of successful collisions, leading to formation of products
2 factors
Energy of collision
geometry of collision
Activation energy
The minimum amount of kinetic energy that particles need in order for a collision to lead to a reaction
Necessary for overcoming repulsion between molecules and for breaking some bonds in the reactants before they can react
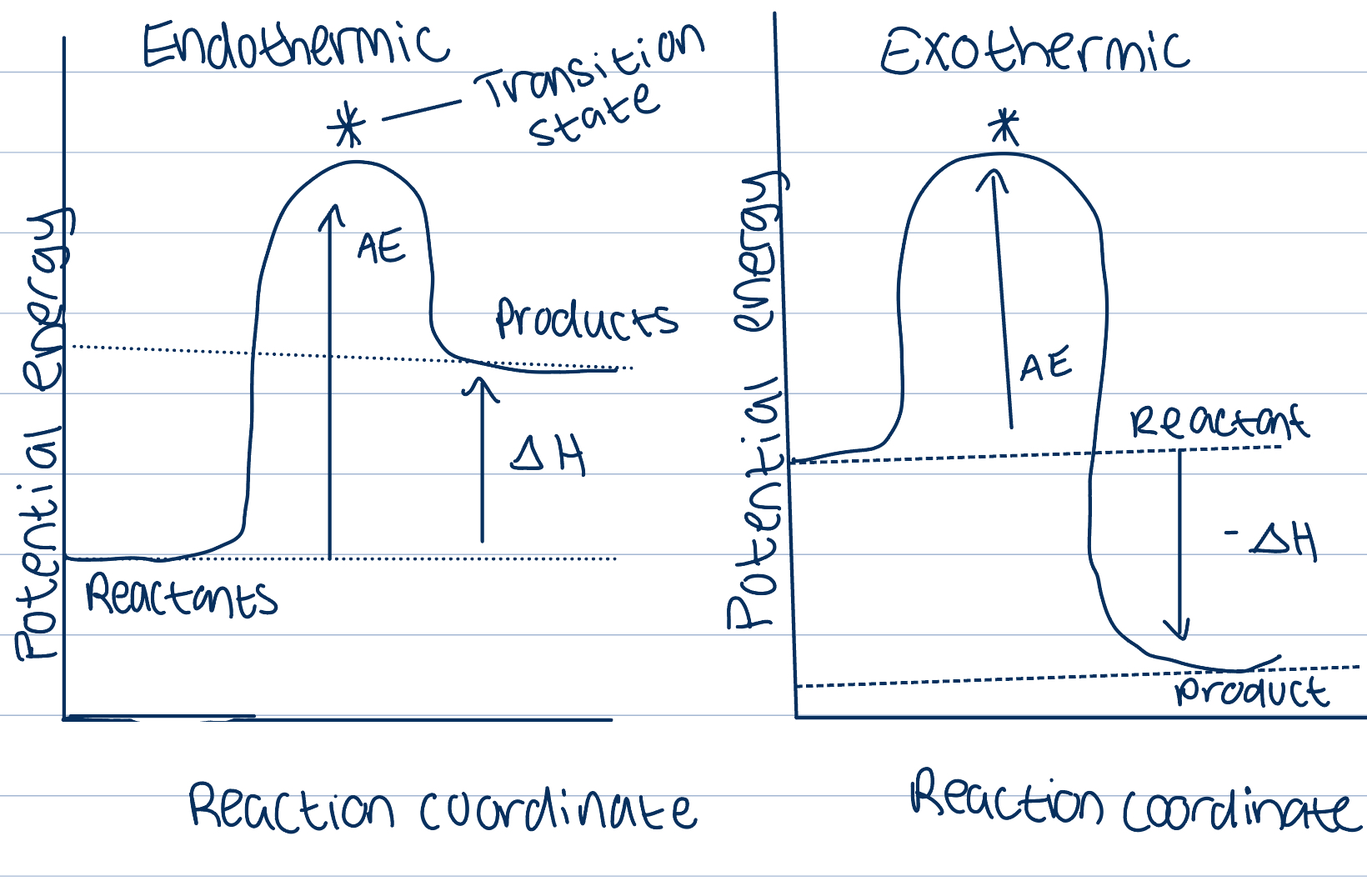
Transition state
The state of energy that reactants must reach before the reaction can occur
Any particles that have KE greater than AE will have successful collisions
Particles with lower values of KE may still collide but these collisions won’t be successful in causing a reaction
ROR depends on proportion of particles that have a KE greater than AE
Magnitude of ROR varies from one reaction to the other, important deciding factor of ROR
Reactions with high AE proceed slower than those with lower AE, as less particles have required AE for a successful collision, will take longer for one to occur
Geometry of collision
Collisions between particles are random, they occur in many different orientations, in some reactions orientation can be crucial in deciding if the collision will be successful and so how many collisions will lead to a reaction
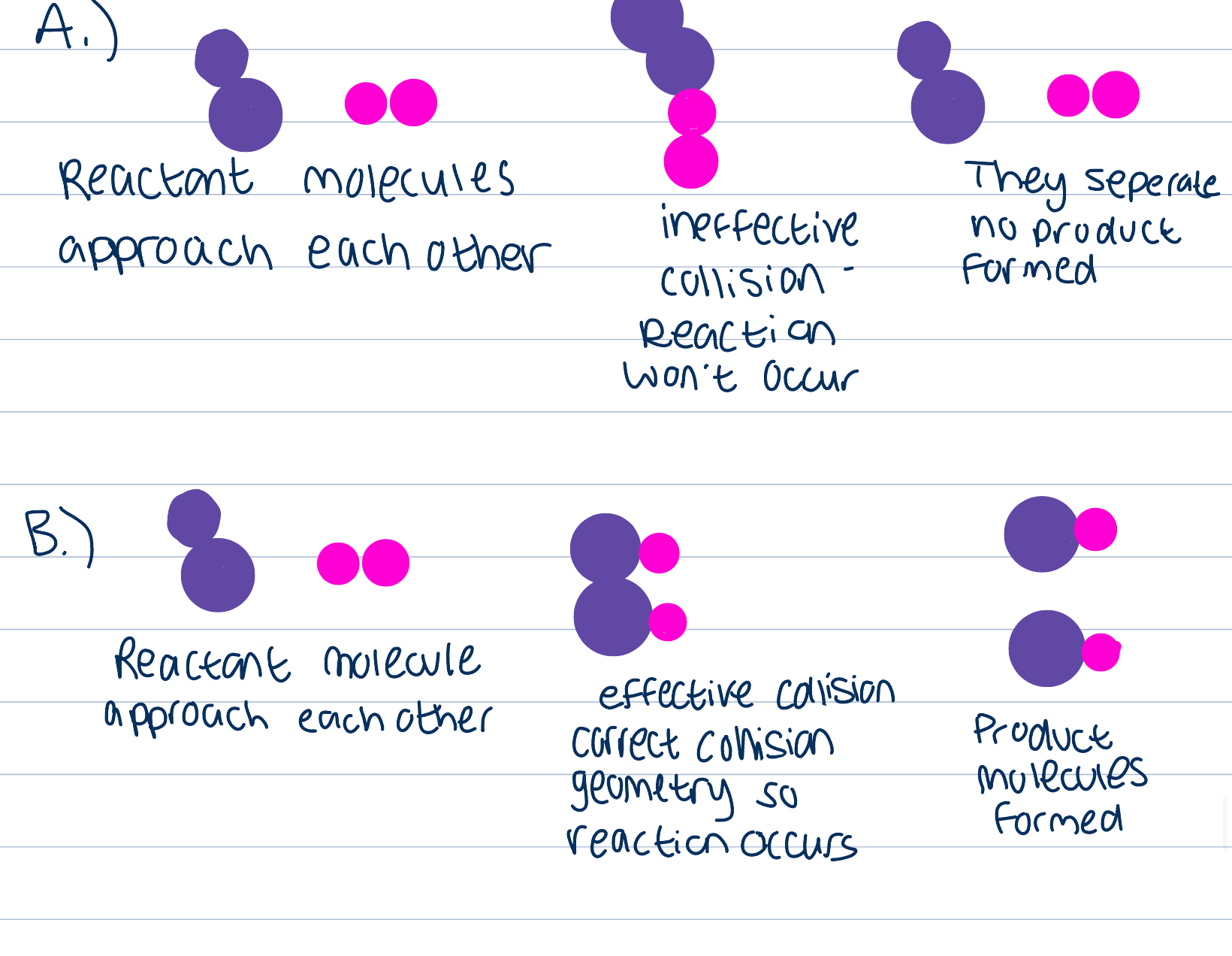
Collision theory
The ROR depends on the frequency of collisions that occur between particles possessing both values of KE greater than AE and appropriate collision geometry
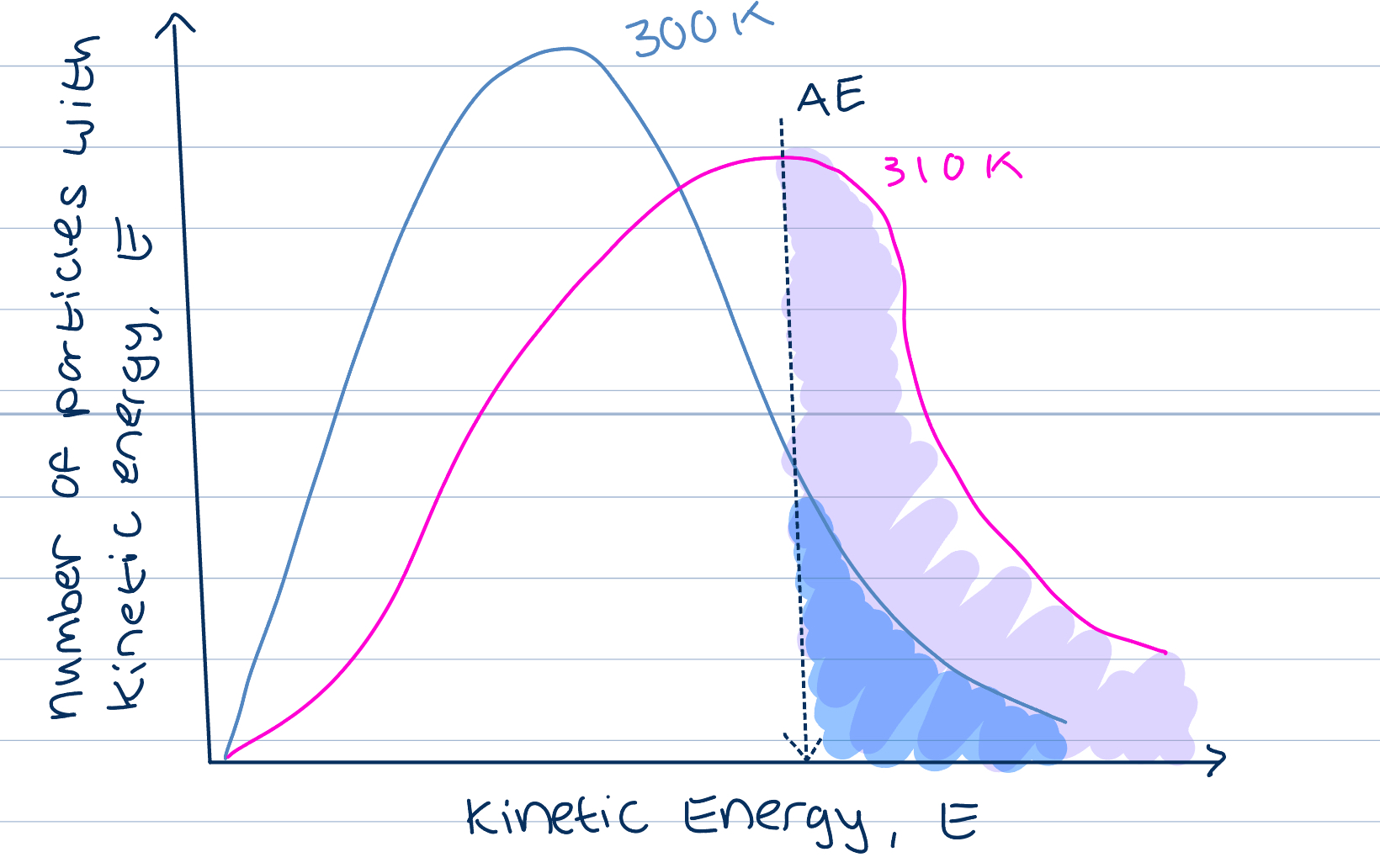
Temperature on ROR
Increase in temp causes increase in KE of particles
Compare the same sample of particles at two different temperatures
Area under the curves the same, same number of particles
AT higher temperature, more particles have higher KE, peak of curve shifts to the right
Shift increases proportion of particles that have KE greater than AE
As temperature increases there is an increase in collision frequency due to increase in KE, but also an increase in collisions involving particles with AE needed to have successful collisions, so increase ROR
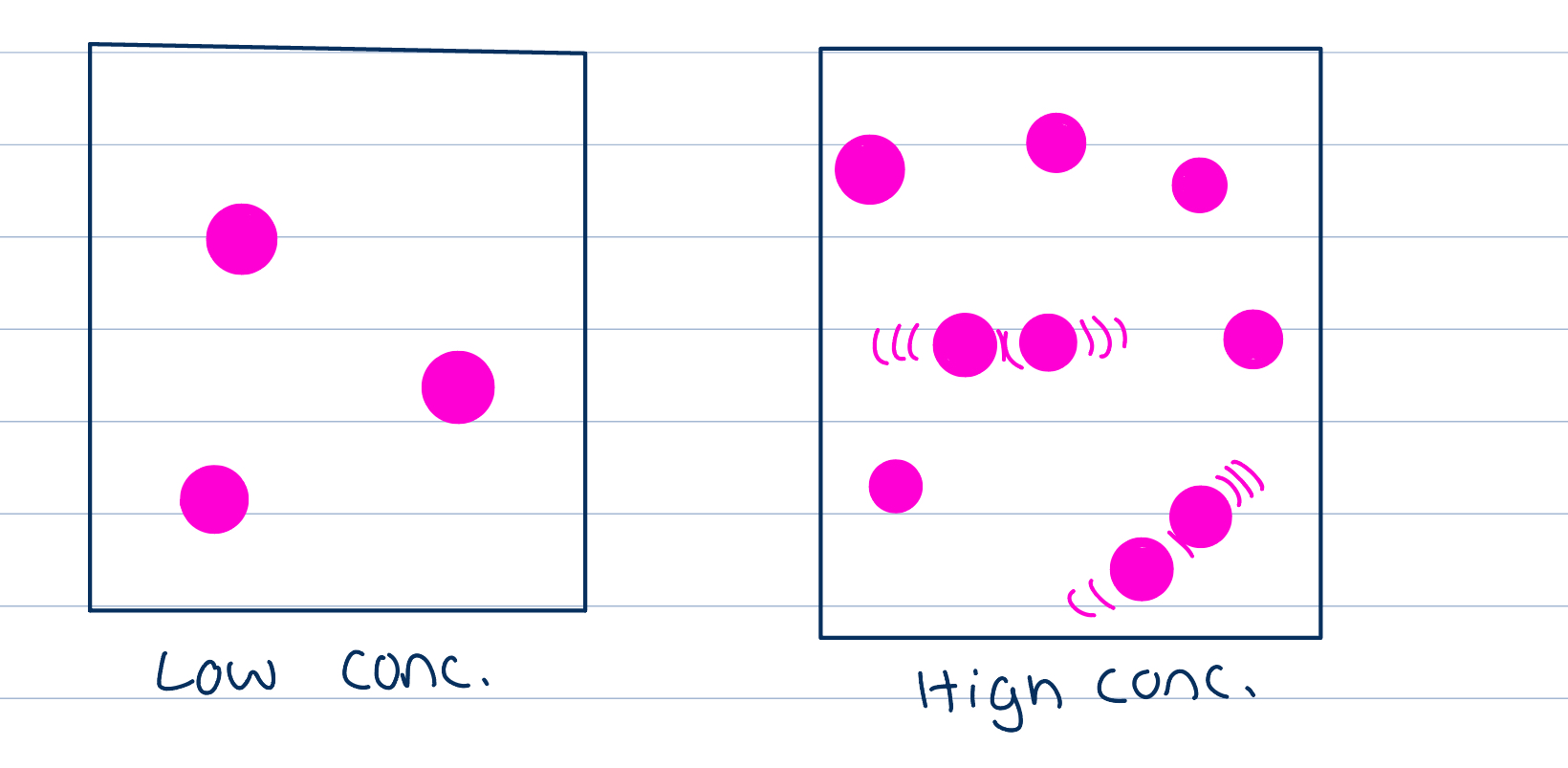
Concentration on ROR
As concentration increases the frequency of collisions between reactants particles increase therefore the frequency of successful collisions increases too, increasing ROR
More particles closer together, greater chance of reacting
As reactants are used up, their concentration falls and the ROR decreases which is why we see curve
Pressure on ROR
Reactions involving gases, increasing pressure increases ROR
higher pressure compresses gas, increasing its concentration, increasing frequency of collisions, increasing chance of successful collisions and ROR
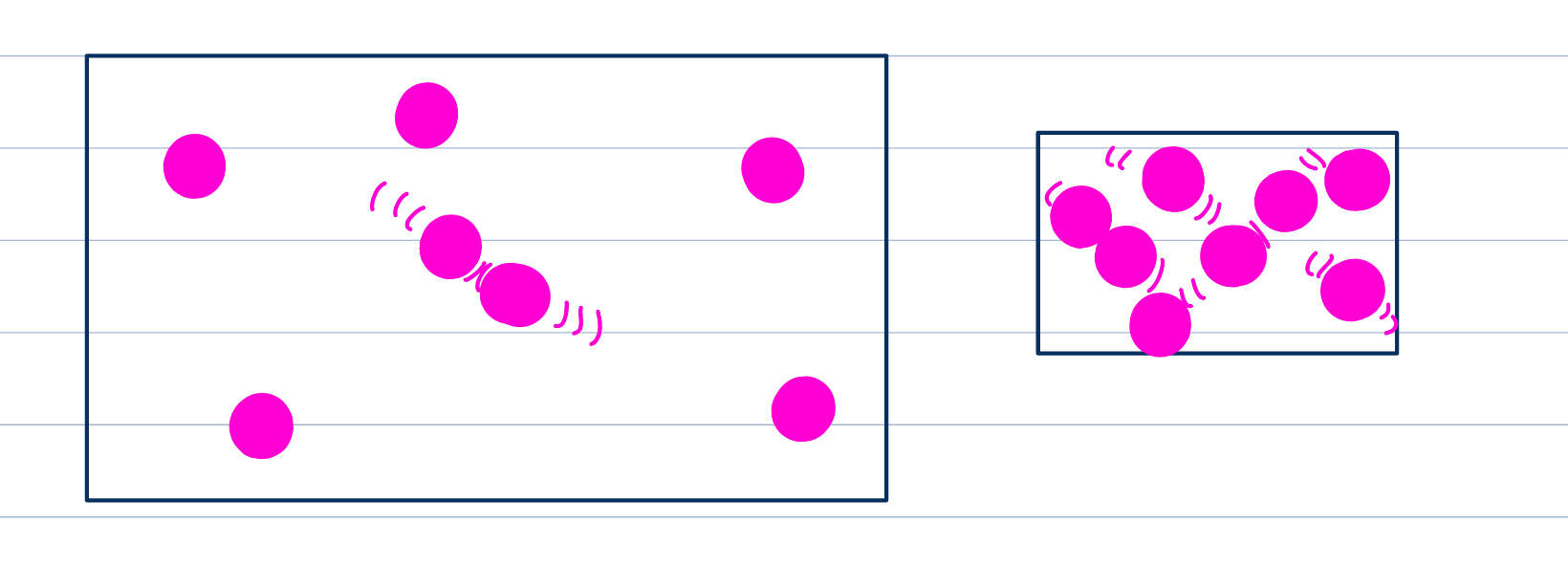
Surface area on ROR
Subdividing a large particle into smaller parts increases the total surface area, allowing more contact and area to collide, higher probability of collisions and successful ones
Like stirring solid particles in liquids to break them up and speed up reaction
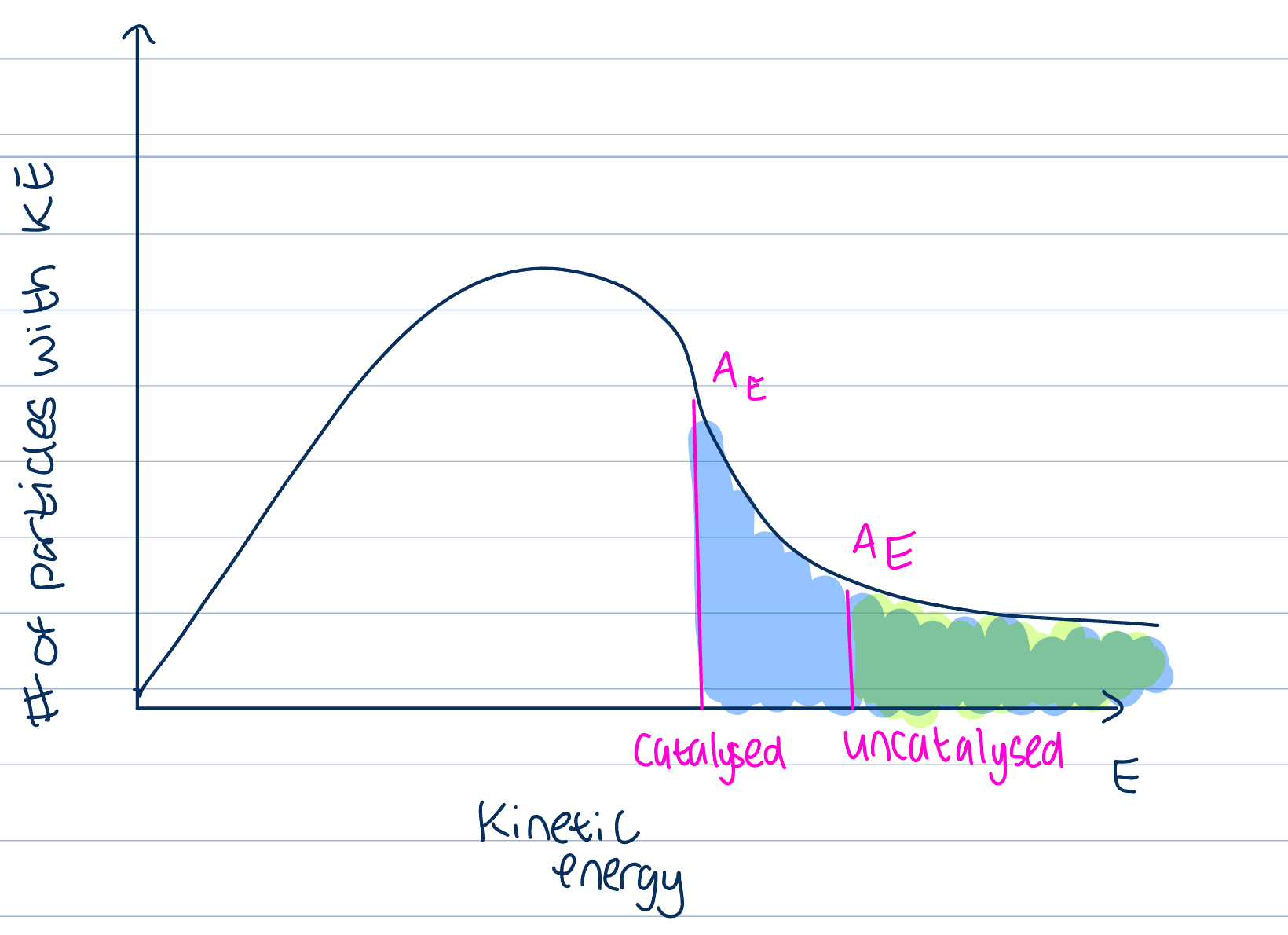
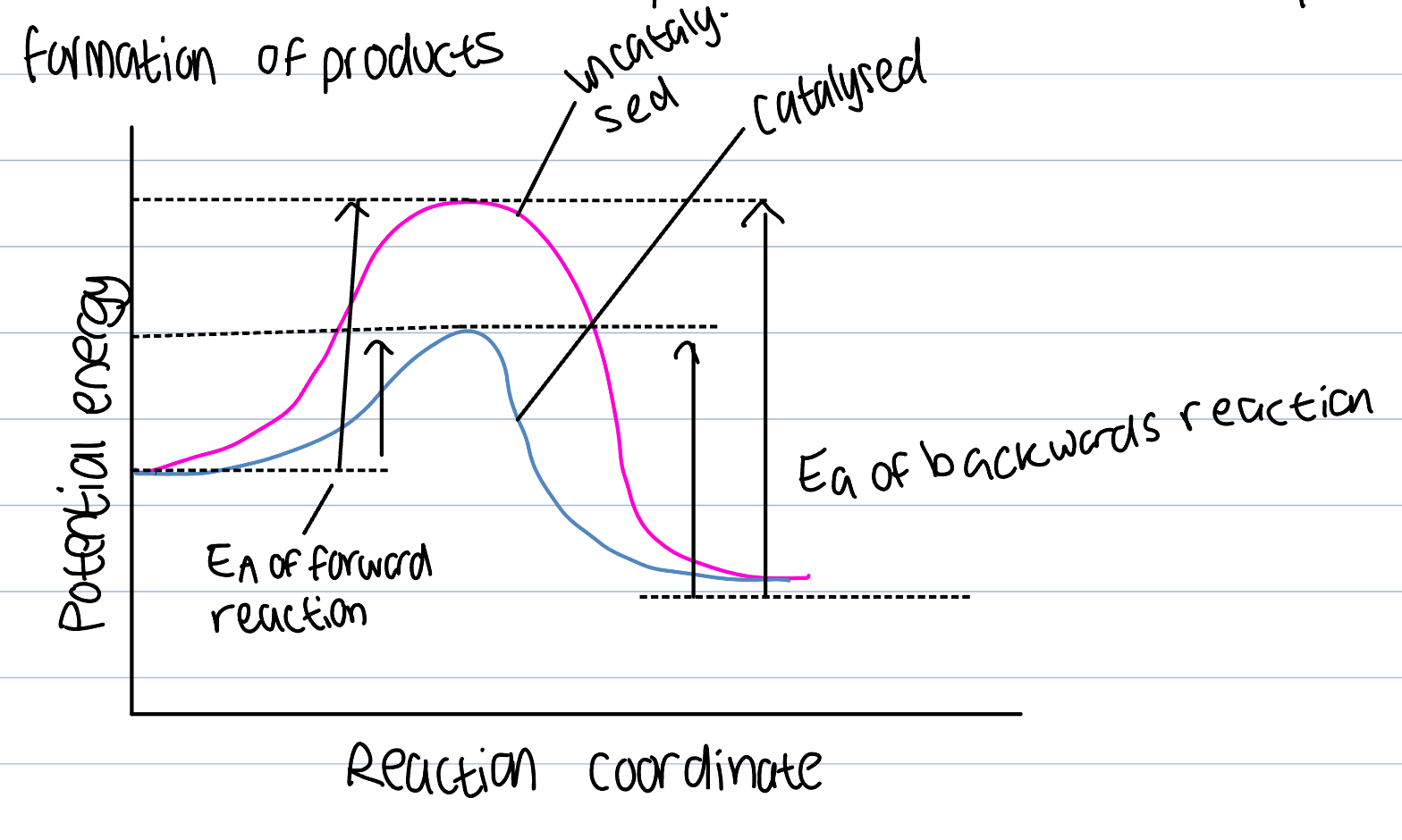
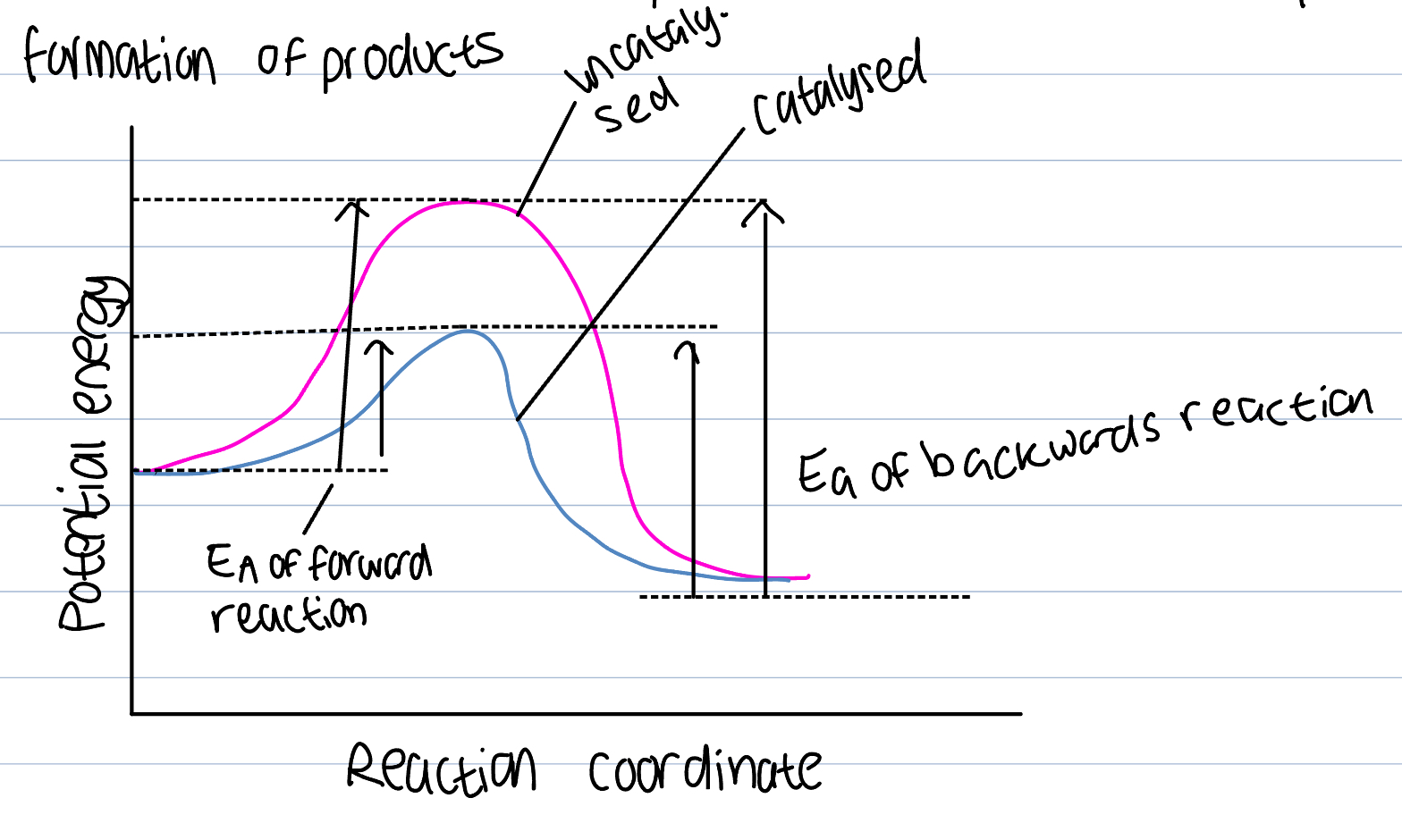
Catalyst on ROR
A substance that increases the rate of reaction without itself undergoing a chemical change
Provides an alternative route for the reaction that has a lesser AE
Larger number of particles will have the KE greater than the AE as catalyst will lower AE necessary and number of successful collisions will increase and ROR increase so product formation will too
Play an essential role in industrial processes, otherwise would proceed too slow and would have to use high temperature to increase ROR
Enzyme
Every biological reaction controlled by a biological catalyst called an enzyme which is specific for each reaction
Good for green chemistry- effective in small quantities, frequently reused, do not make waste, increase atom economy
Controls
When effecting temperature effect on ROR keep pressure, concentration, surface area constant
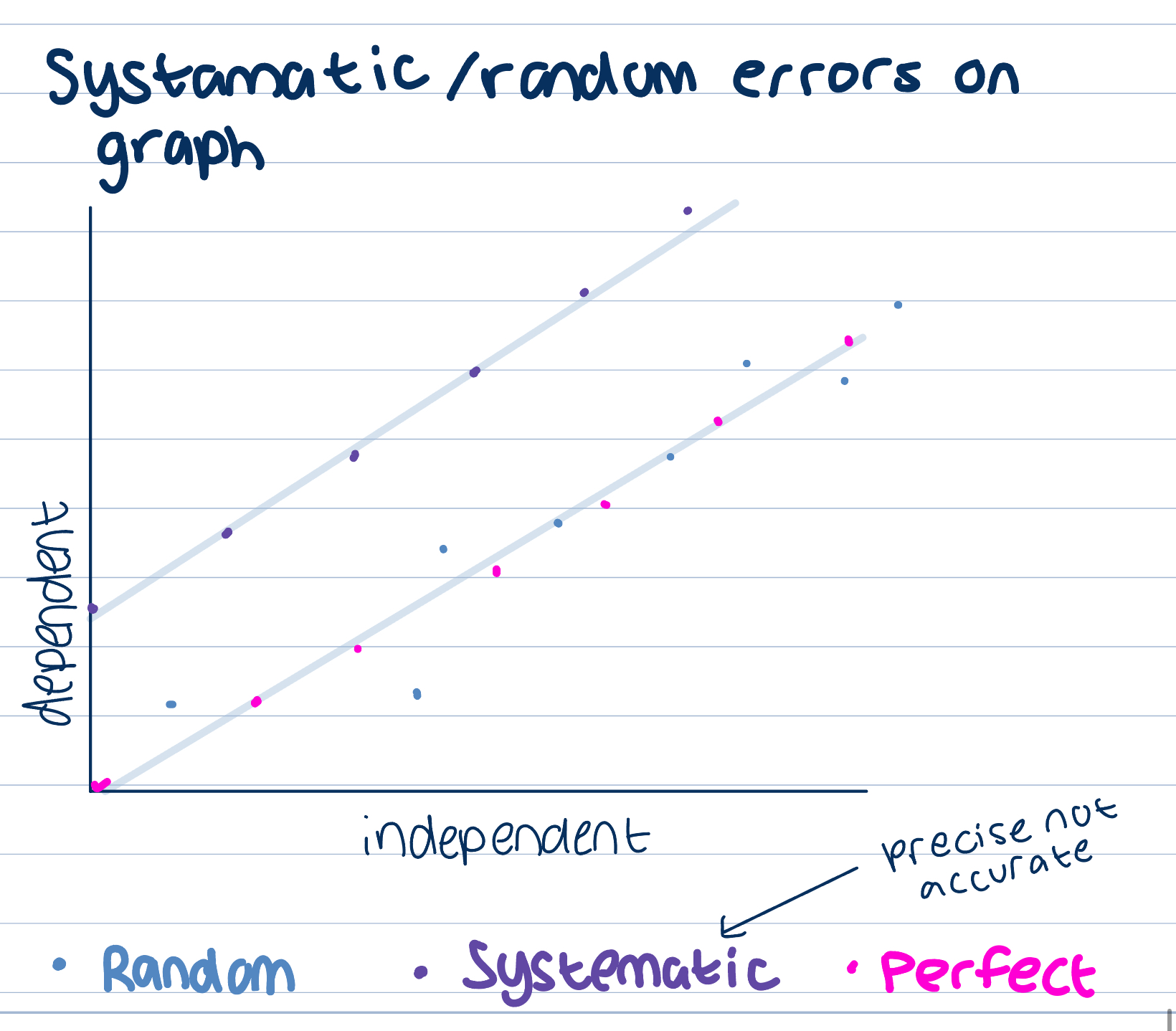
Graph errors
Systematic errors when all values higher than expected, precise not accurate errors in method
Random errors due to measurement equipment limitations, changes in surroundings, not enough repeats, accurate not precise
Bromine example physical system
Brome has a boiling point close to room temp so many particles have enough energy to form vapour in evaporation but as the container is sealed and vapour cannot escape, its concentration will increase and some of the vapour molecules collide with the surface of the liquid, lose energy, become liquid in condensation.
liquid to gas- evaporation
gas to liquid- condensation
Rate of condensation will eventually equal evaporation as an increase in concentration of vapour will cause more vapour to collide with the surface of the liquid (condensation)
At this point no net change in amount of liquid and has present
Equilibrium reached
Only occurs in closed system where bromine cannot escape as valour and must condense back
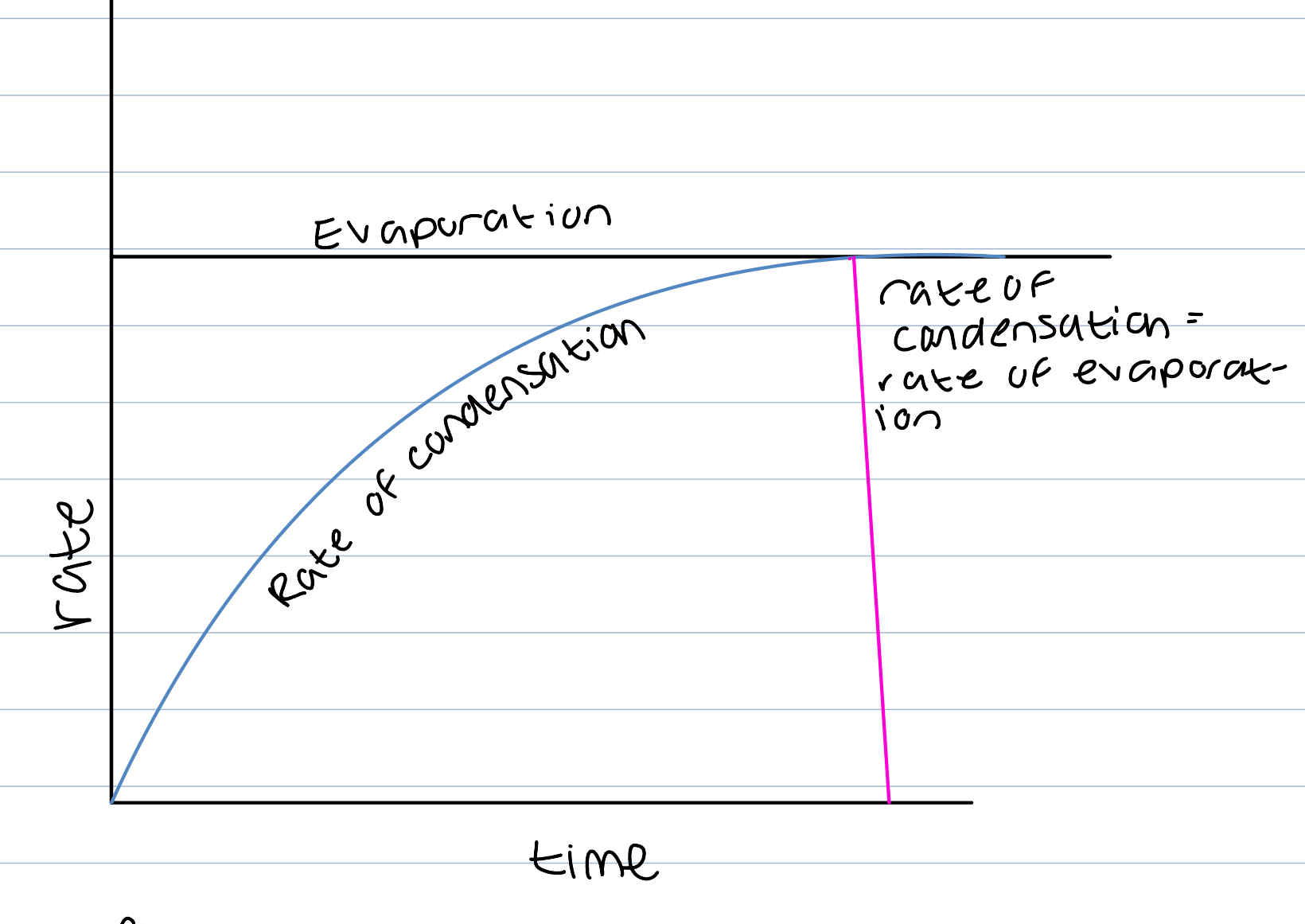
Characteristics equilibrium
Dynamic- Both forward and backward reactions are still occurring at same rate
Closed system- No exchange of matter with surroundings, reactants and products can react and recombine
Concentrations remain constant- Produced and destroyed at same rate
No change in observable properties- colour and density will not change as they depend on concentration
Either direction- Same equilibrium mixture result if under same conditions no matter if reaction starts with all reactants, all products, or mix
Chemical systems, 2Hi → H2 + I2
I2 is a purple gas
Start with 2HI in a sealed container
Increase in purple colour as I2 is produced
After awhile, increase in colour stops
Rate of disassociation of HI is fastest in start when concentration of HI is greatest and falls as reaction goes on
Reverse reaction (association of H2 and I2 to make 2HI) which initially is zero because no H2 or I2 produced and then speed up when concentrations of H2 and I2 increase
Rate of association = rate of disassociation so concentrations remain constant and colour in flask remains the same
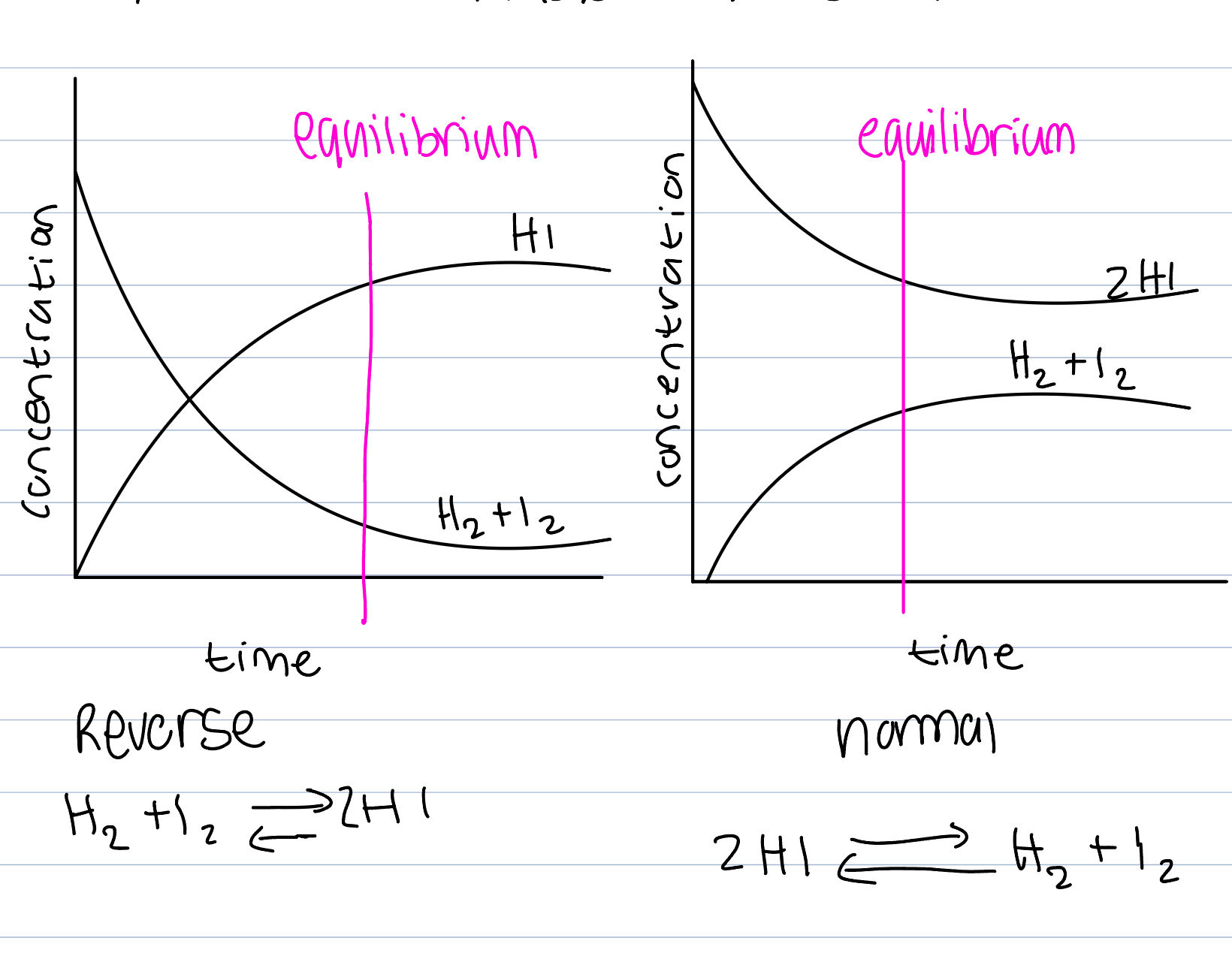
Forwards vs backwards reaction
Reactants to products is forwards
Products to reactants is backwards
Equilibrium position
Proportion of reactant and product in mixture
Mixture contains more products (higher conc.) then it lies to the right
Mixture contains more reactants (higher conc.) then it lies to the left
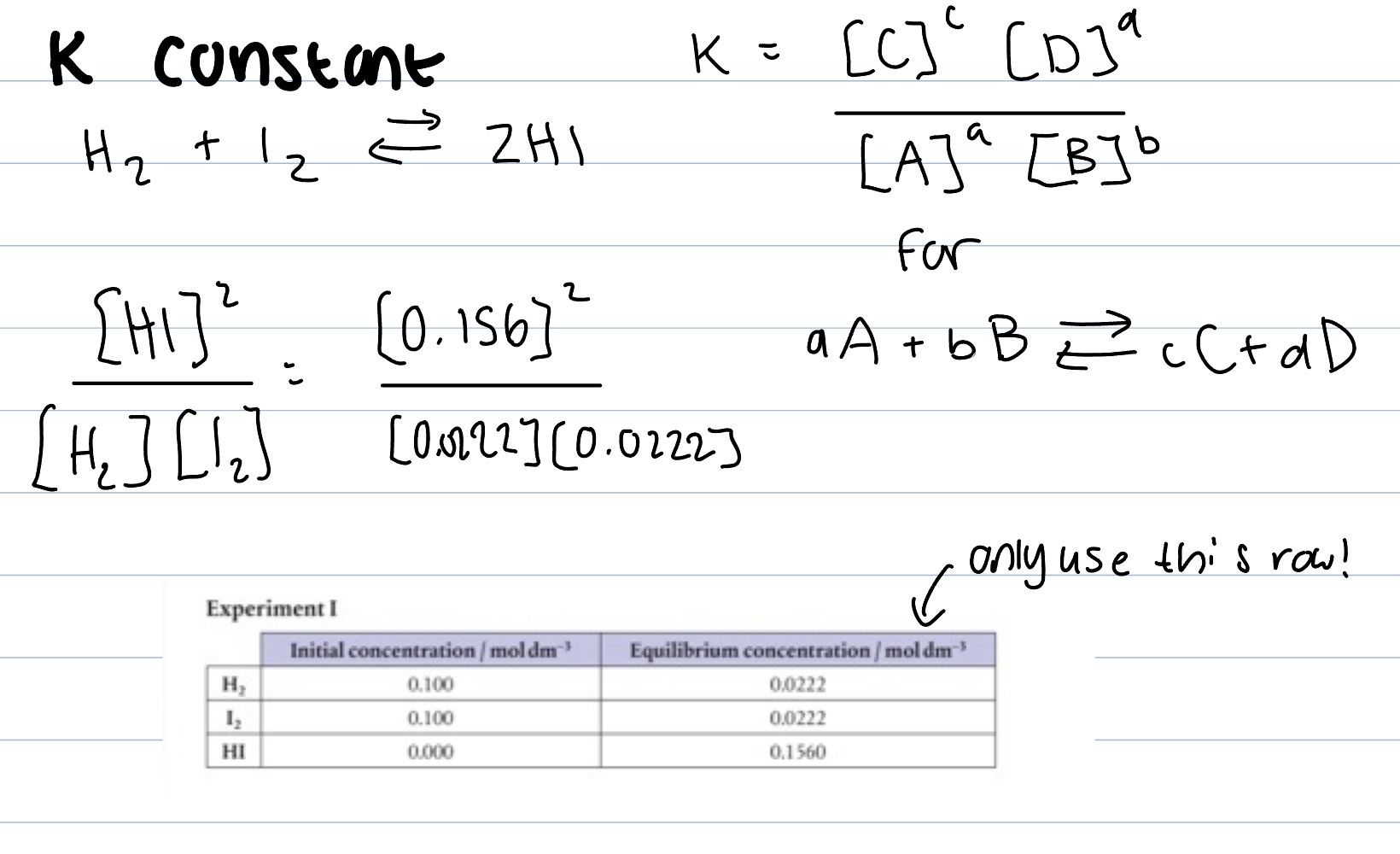
K constant
Fixed value for a reaction at a set temp
Only use for equilibrium concentration
Products over reactants
High value of K means more products than reactants, lies to the right, almost goes to completion
Low value of K means more reactants than products, lies to the left, does not go to completion
equals one, significant amount of products and reactants at equilibrium
equilibrium constant
The equilibrium constant for one reaction is the reciprocal for its reverse reaction (products and reactants switched )
Weak vs strong acids
Weak acids go very little disassociation ( low K value )
Stronger acids more disassociation (high K value)
Normally lies left for acid disassociation
La chaterliers principle
A system at equilibrium that is subjected to change will respond in such a way to minimise the effect of that change
Whatever we do to a system, it will respond in the opposite way
After awhile a new equilibrium will be established with a different composition to previous mixture
Changes in concentration
Increase in concentration in one of the reactants
Cause rate of forward reactions to increase and backwards not effected, reactions rates now not equal
Equilibrium will shift in favour of products
New concentrations of reactants and products
Equilibrium re establishes itself
Value of K unchanged
Vice versa, decrease in concentration of product, shift equilibrium in favour of product, forwards reaction
New equilibrium position
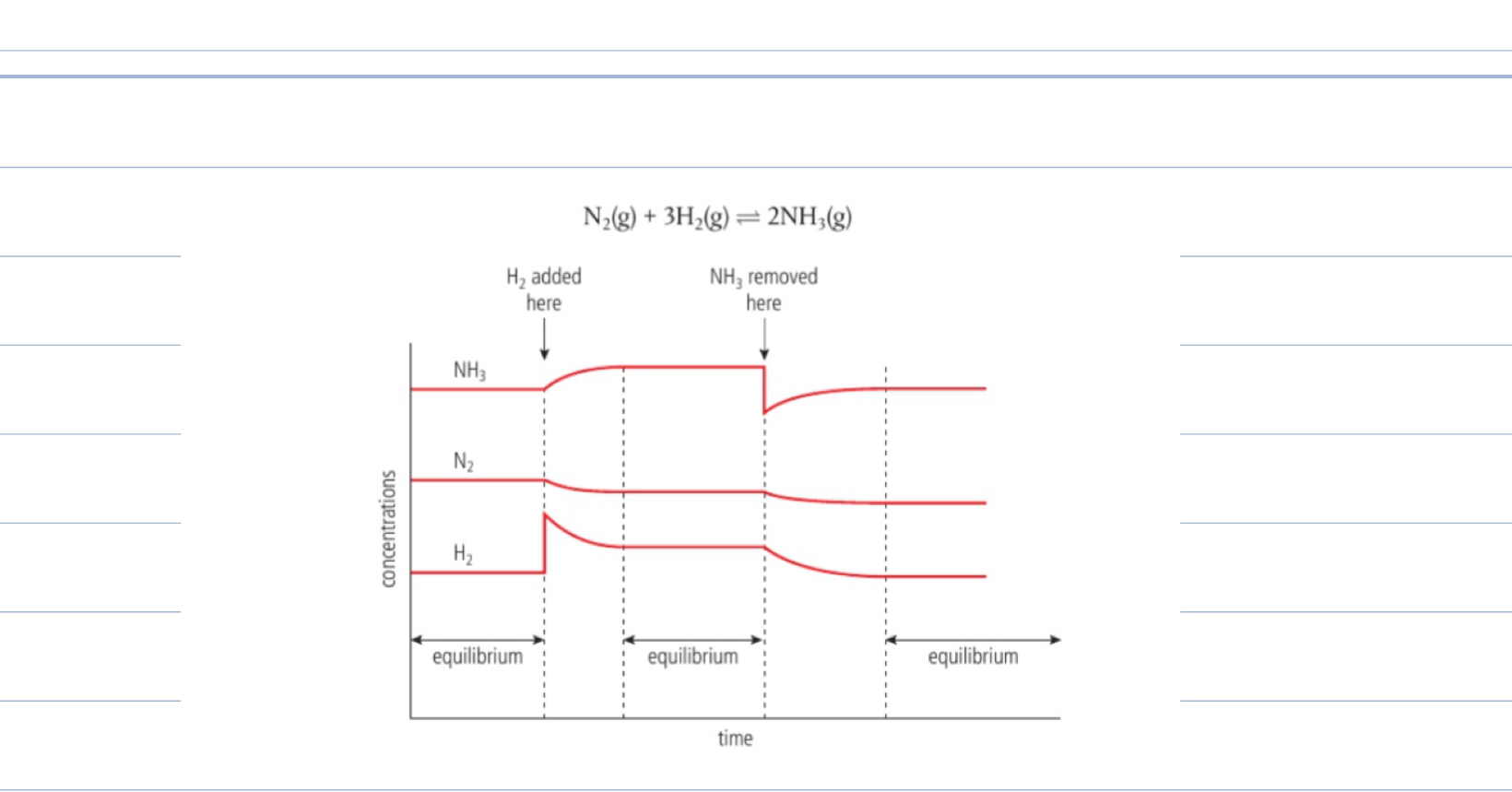
In industrial processes
Products will be removed as soon as they form to ensure equilibrium is pulled to the right which will increase yield of product
Changes in pressure
Equilibria involving gases will be effected by a change in pressure if reaction involves change in number of molecules
If reaction is subject to an increase in pressure , system decreases this pressure by favouring side with smaller number of molecules, vice versa if decrease in pressure, favour side with larger number of molecules
temperature doesnt change so K value stays same
Count molecules by coefficient numbers of reactants vs products, which has more
Changes in temperature
K is temperature dependent, changing temp will change K
Exothermic releases energy, -H
endothermic absorbs energy, +H
Negative sign for enpalthy means forward reaction is exothermic and heat is a product. Therefore if you increase heat, reaction wants to make less, so endothermic reaction favoured ( K decreases as you have more reactants than products). decrease temp, wanna make more, exothermic reaction favoured (value of K increases as products more than reactants)
Positive sign for enpalthy means forward reaction is endothermic and heat is a reactant, so if temperature increases you wanna make less so forward reaction, endothermic is favoured, (K value gets higher as you make more products than reactants). if heat is decreased, then backward reaction, exothermic is favoured as you wanna make more, K value lowers (more reactants than products)
Addition of a catalyst
Because the forward and backward reactions pass through same transition state, catalyst lowers activation energy by the same amount for both
No effect on position of equilibrium or K value
Speeds up attainment of equilibrium and so cause quick formation of products
Used in processes to speed up Rate of product formation
Everything only in gaseous steam or heterogeneous mixture with reactants and products in liquid and gaseous states
Haber process
Based on N2+2H2 →← 2NH2 -93 kJ/mol.
Maximise conversion of reactant to product
Concentration- Increase concentration of reactants, or decrease concentration of products while it forms to switch equilibrium to the right
Pressure- High pressure to shift equilibrium right (less molecules on right)
Temperature- Low temperature, exothermic reaction favours, this causes low ROR so do not make temp too low
Catalyst- Used to speed up reaction and make up for low temperature
Haber process only achieves conversion of reactants to products of 10-20 percent per pass through reactor
Unconverted reactants recycled to reactor to obtain a 95 percent yield
Prevent and minimise waste for low equilibrium yield
concentration is constant but not
equal