Barron's Bio unit 1: properties of water and macromolecules
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
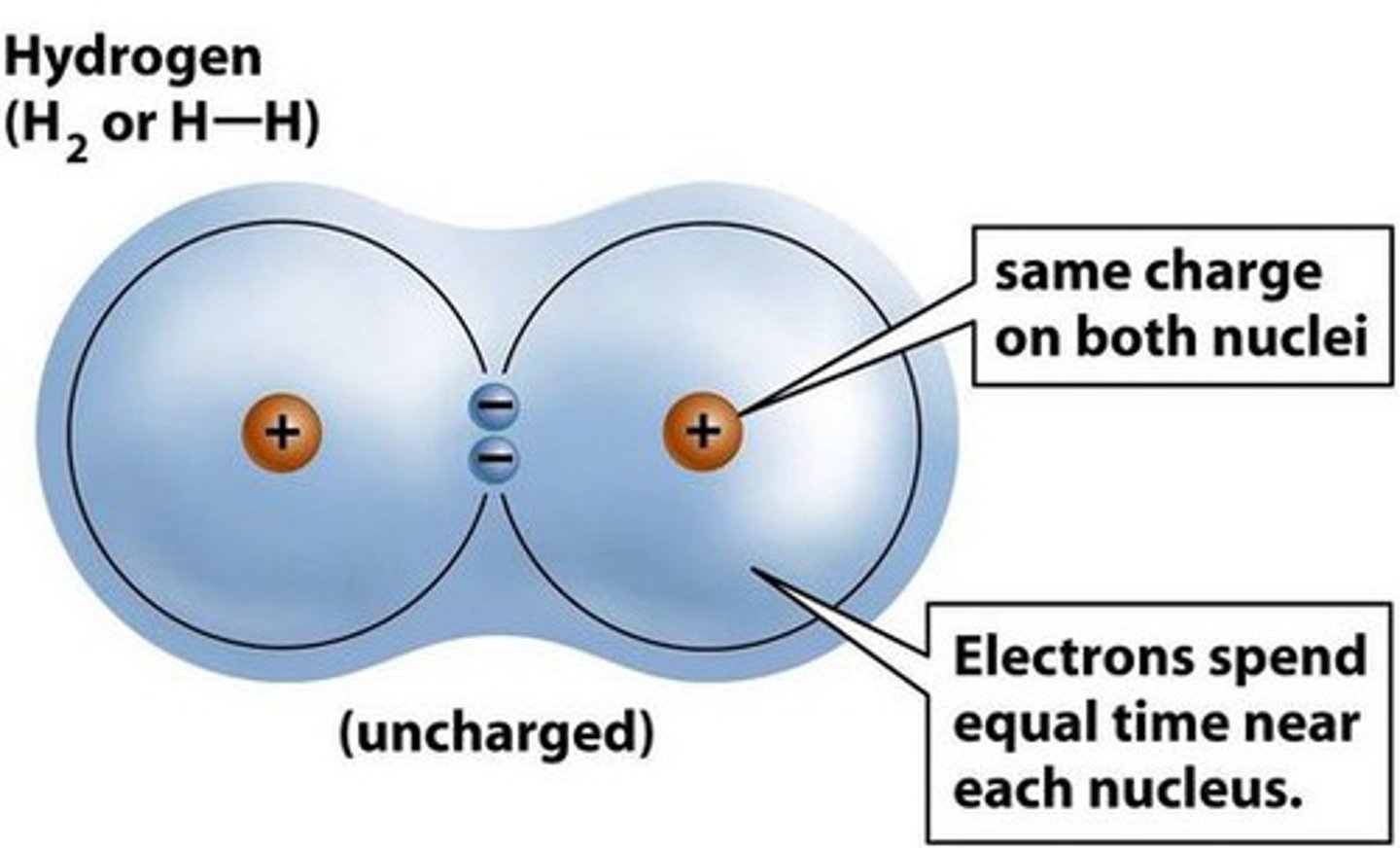
covalent bond
A bond between atoms in which the electrons are shared
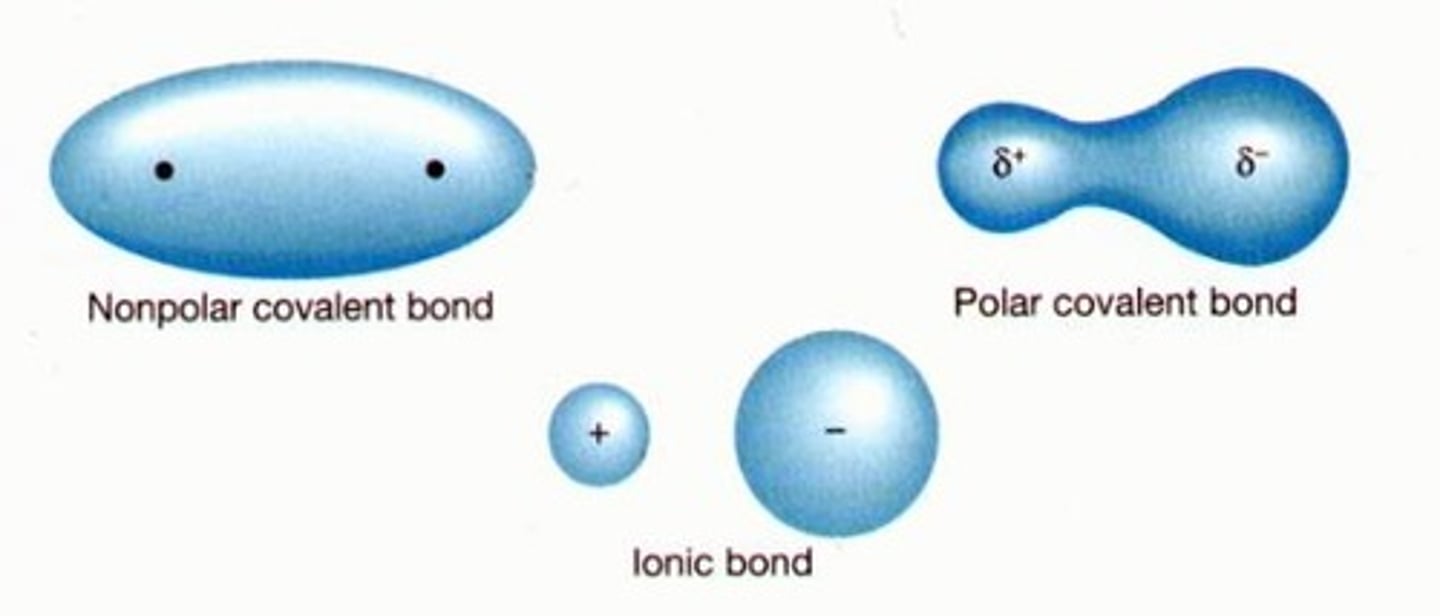
ionic bond
a bond between atoms that involves a transfer of valence electrons from the less electronegative atom to the more electronegative one.
Nonpolar covalent bond
A covalent bond in which the 2 atoms equally share electrons and the electronegativity difference between those atoms is minimal
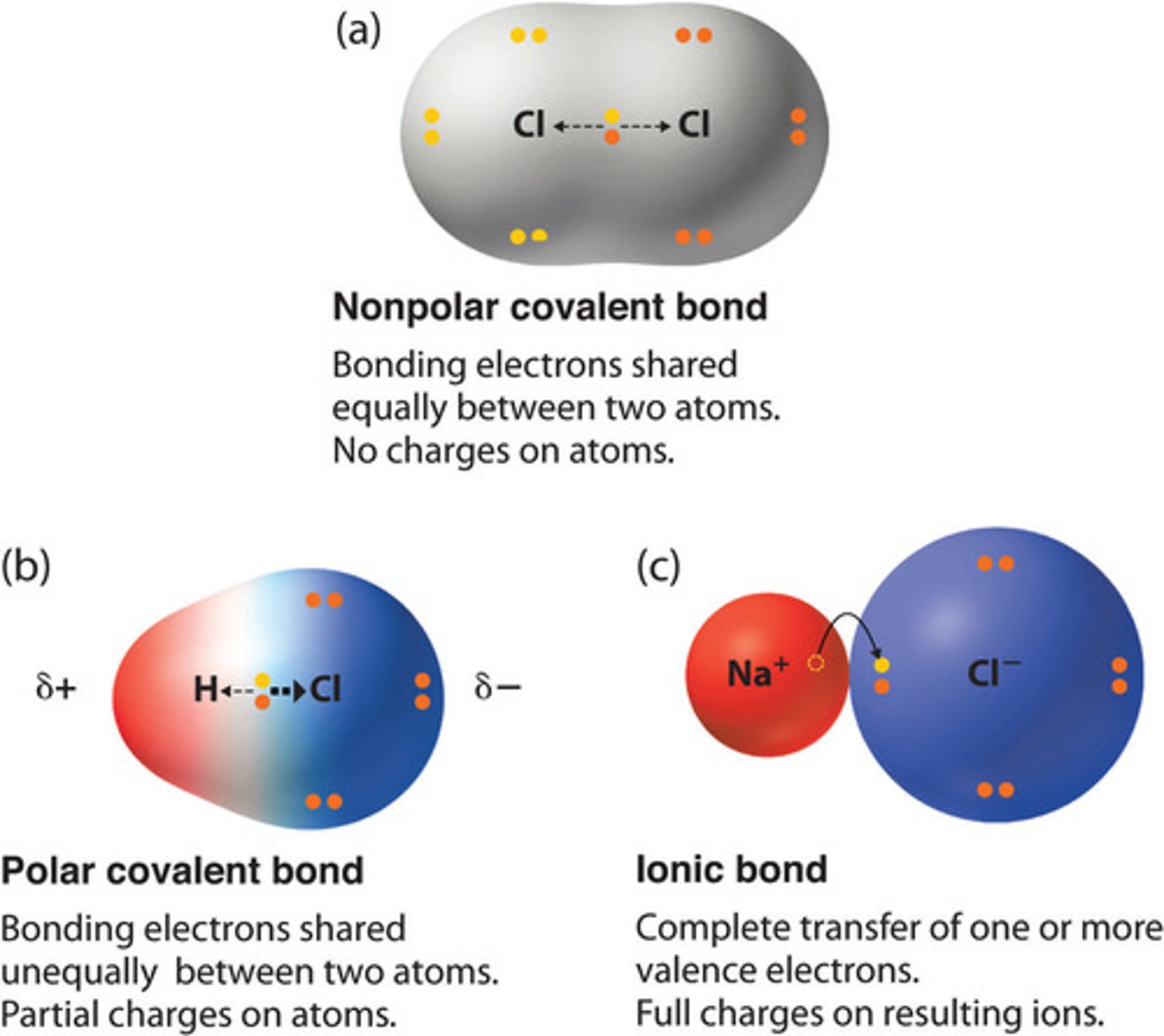
Polar covalent bond
A covalent bond where the electrons are not shared equally between atoms as they differ significantly in electronegativity
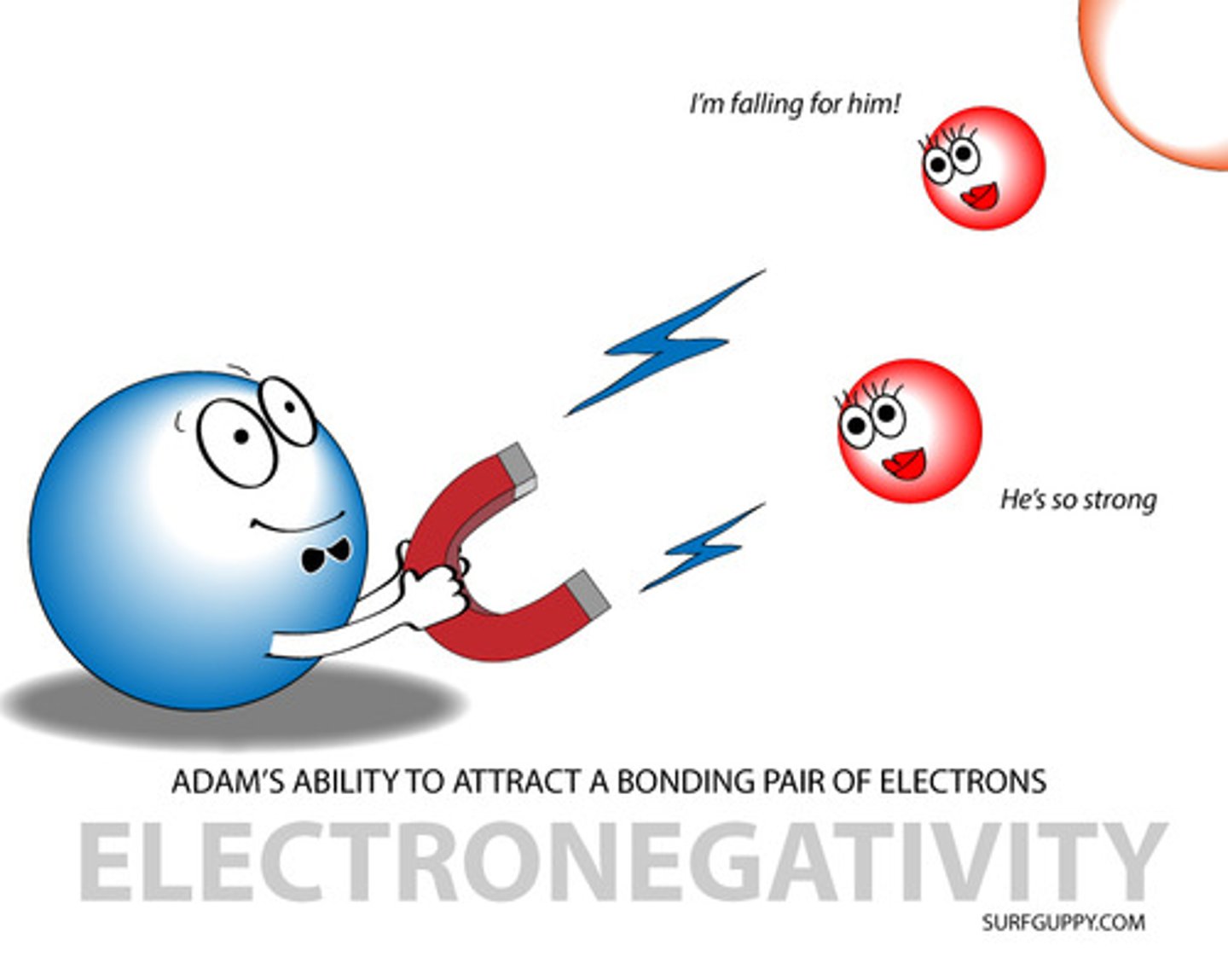
Electronegativity
the ability of an atom to attract electrons.
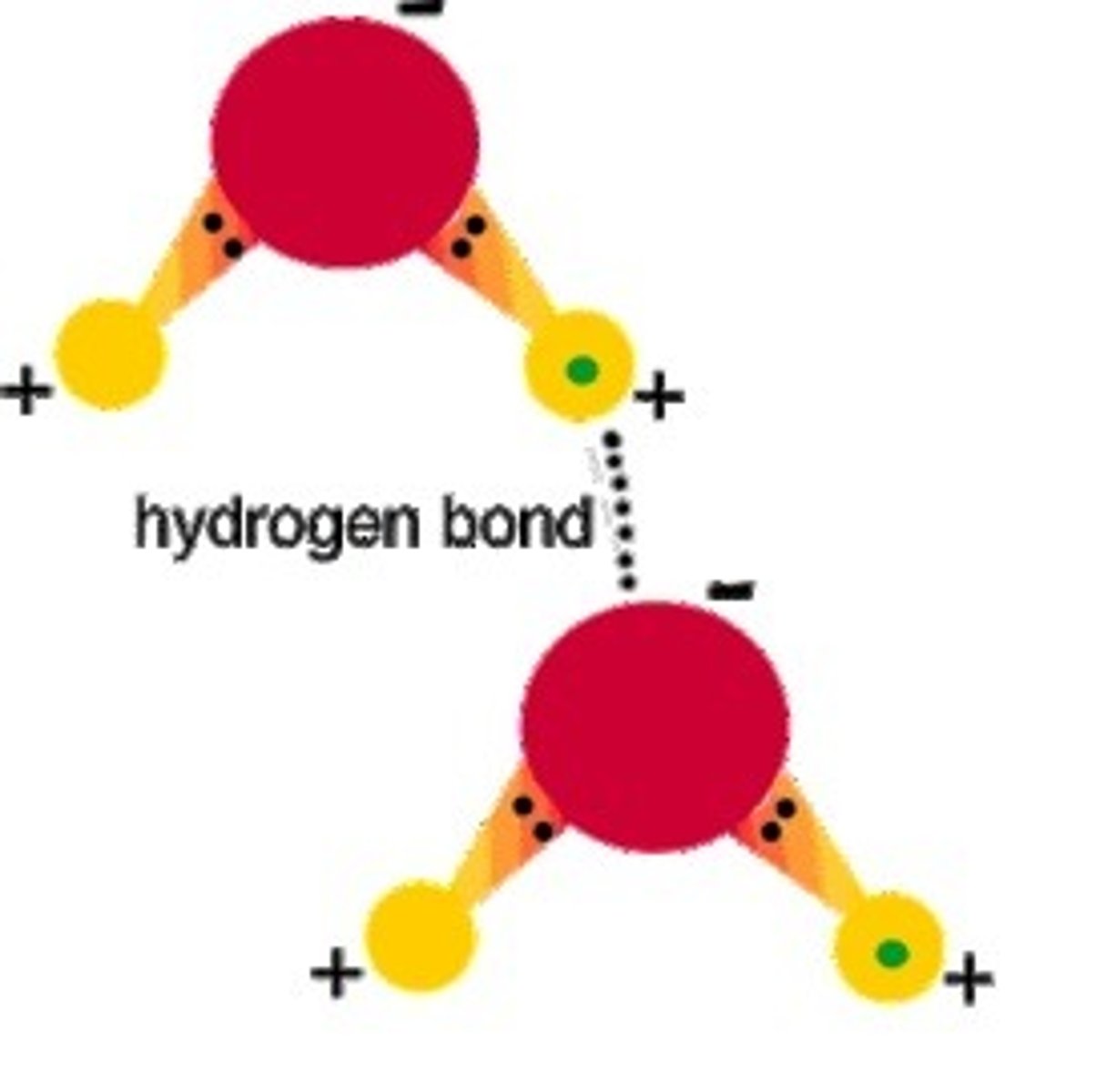
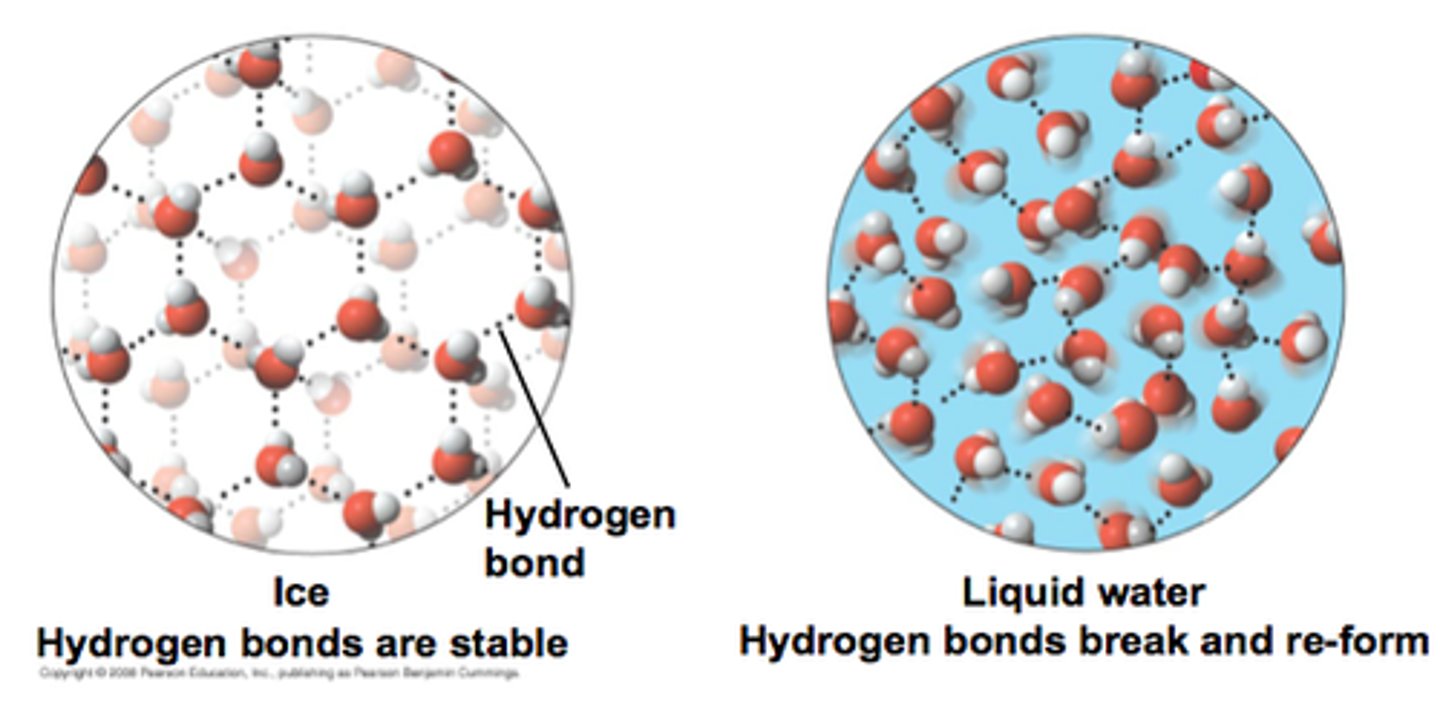
hydrogen bond
The intermolecular electrostatic attraction that occurs between a hydrogen atom that is bonded to a more electronegative atom on one molecule and a more electronegative atom on a different molecule.
Adhesion
the attraction of a molecule to a different type of molecule
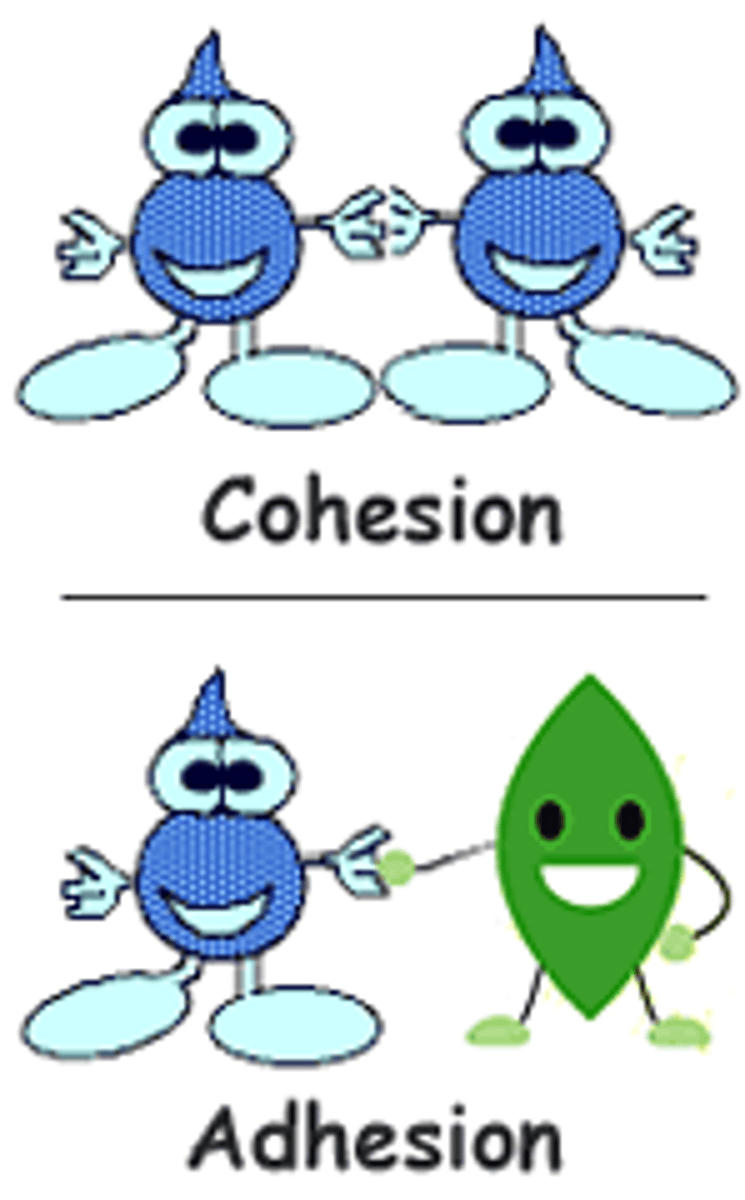
Cohesion
attraction of molecules of the same type of molecule
Capillary Action
The movement of water up a narrow tube because of its adhesive and cohesive properties
Specific heat
The quantity of heat required to raise the temperature of one gram of substance to one degree celsius. Water has high SH

Density of ice
Due to water's ability to form hydrogen bonds, the crystalline structure of ice takes up more volume than an equivalent mass of liquid water and the density of ice is less than the density of water.
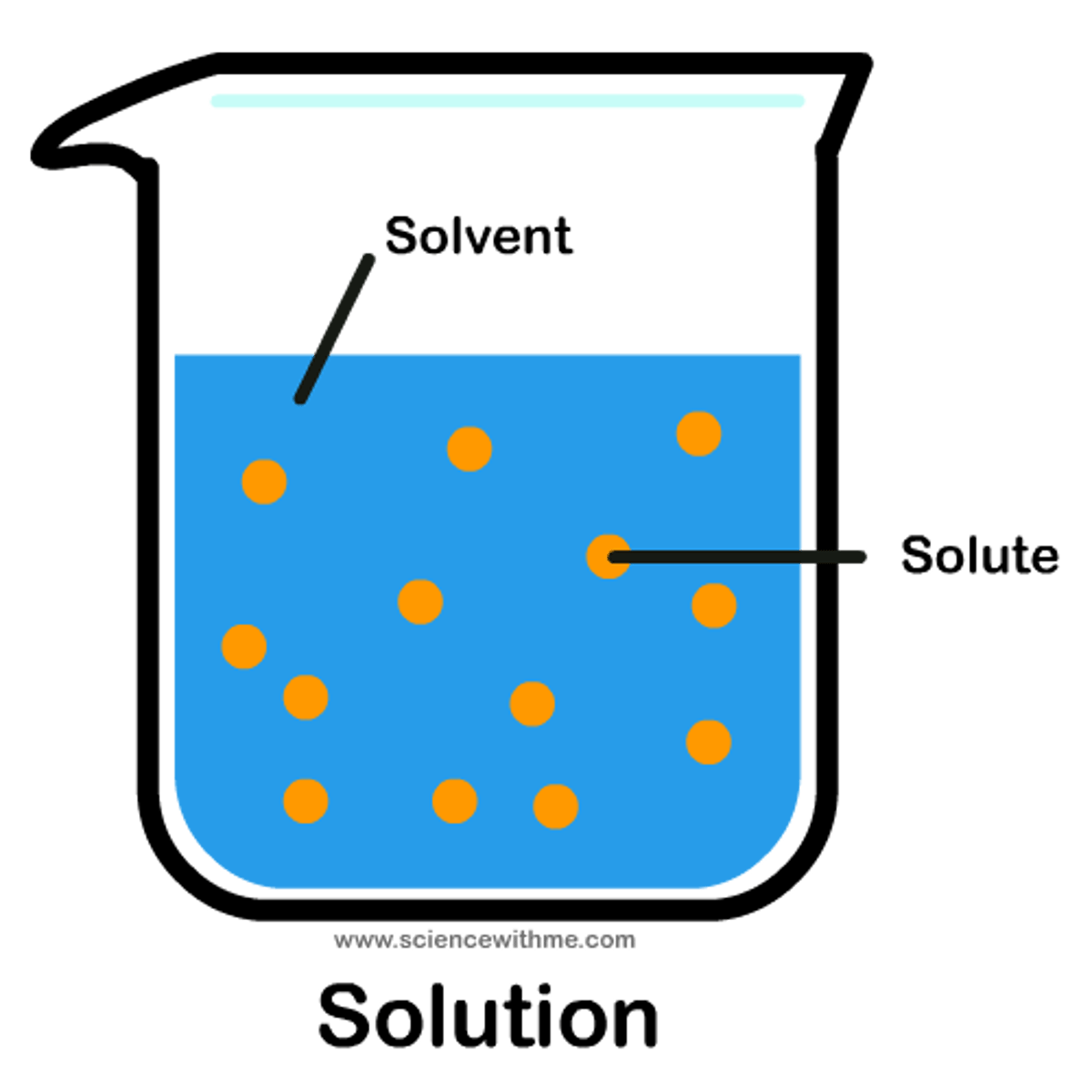
Solvent
the liquid in which a solute is dissolved. Water's ability to form hydrogen bonds makes it a good solvent for ions and polar molecules.
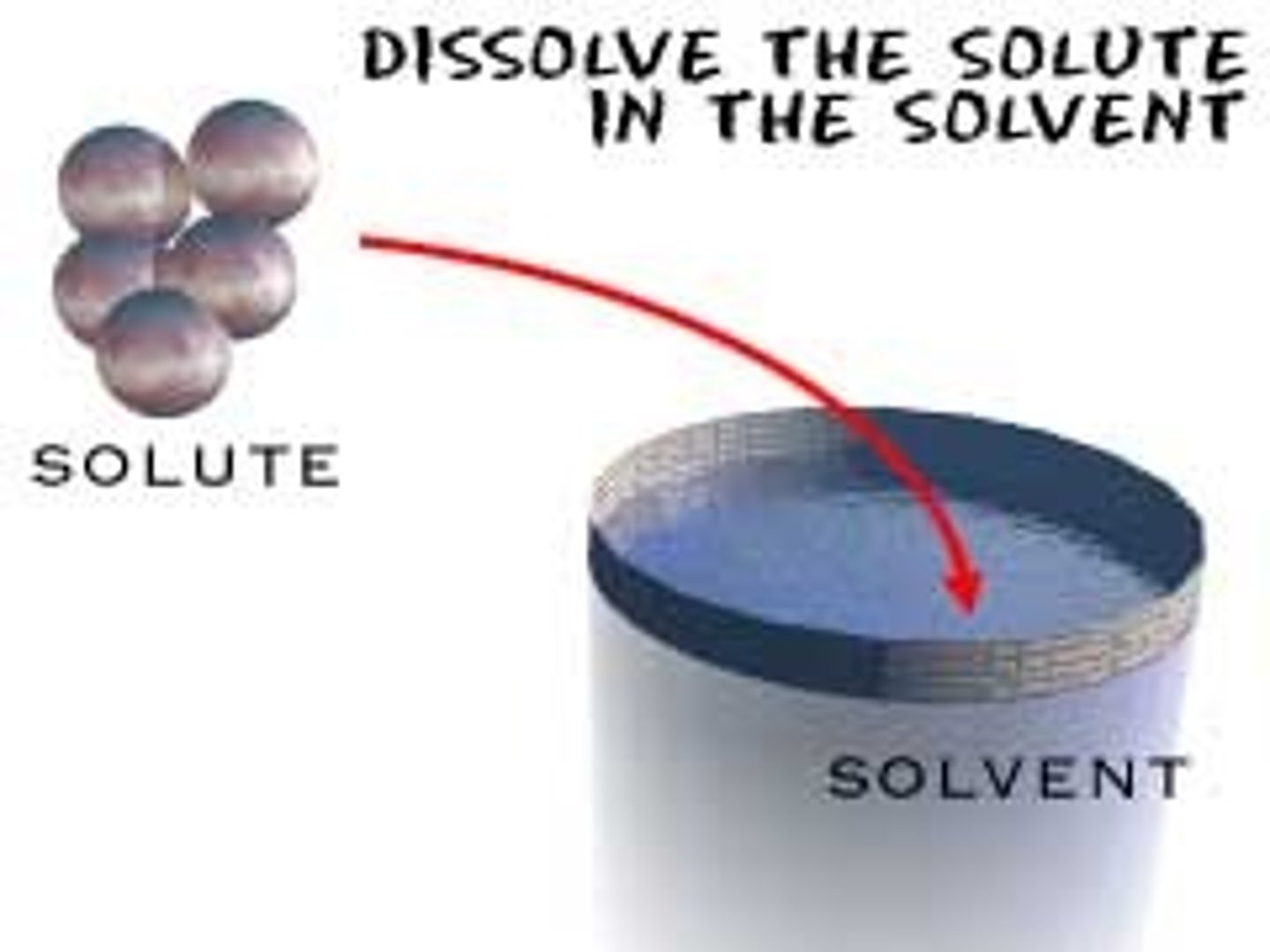
Solute
A substance that is dissolved in a solution.
pH
As the concentration of hydrogen ions increases, the pH decreases.
buffer
a solution that resists changes in pH. Buffers are important for maintaining homeostasis.
surface tension
the tension of the surface of a liquid caused by its cohesive properties. Water's hydrogen bonds lead t water's high cohesive properties and high surface tension.
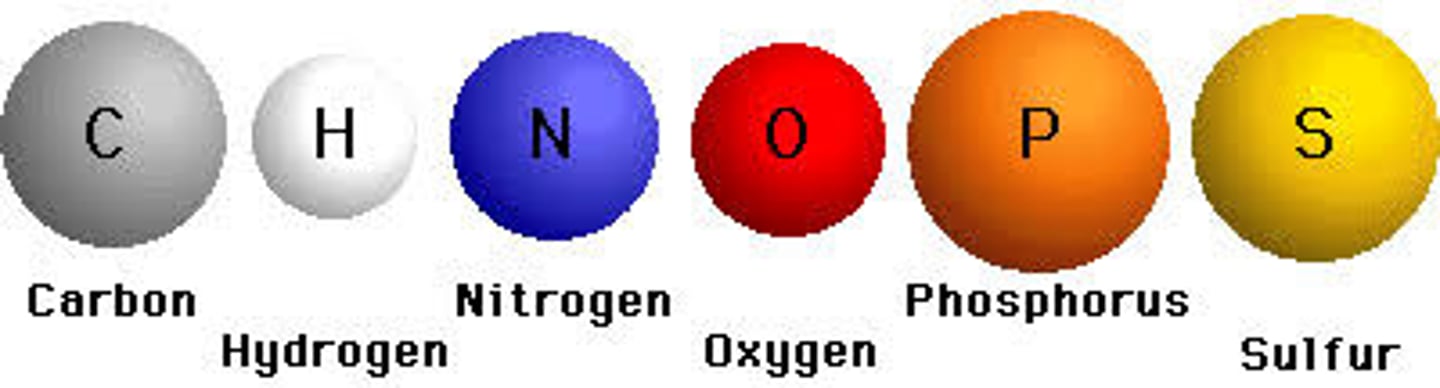
CHOPNS
the elements most frequently found in biological molecules: Carbon, Hydrogen, Oxygen, Phosphorous, Nitrogen, and Sulfur
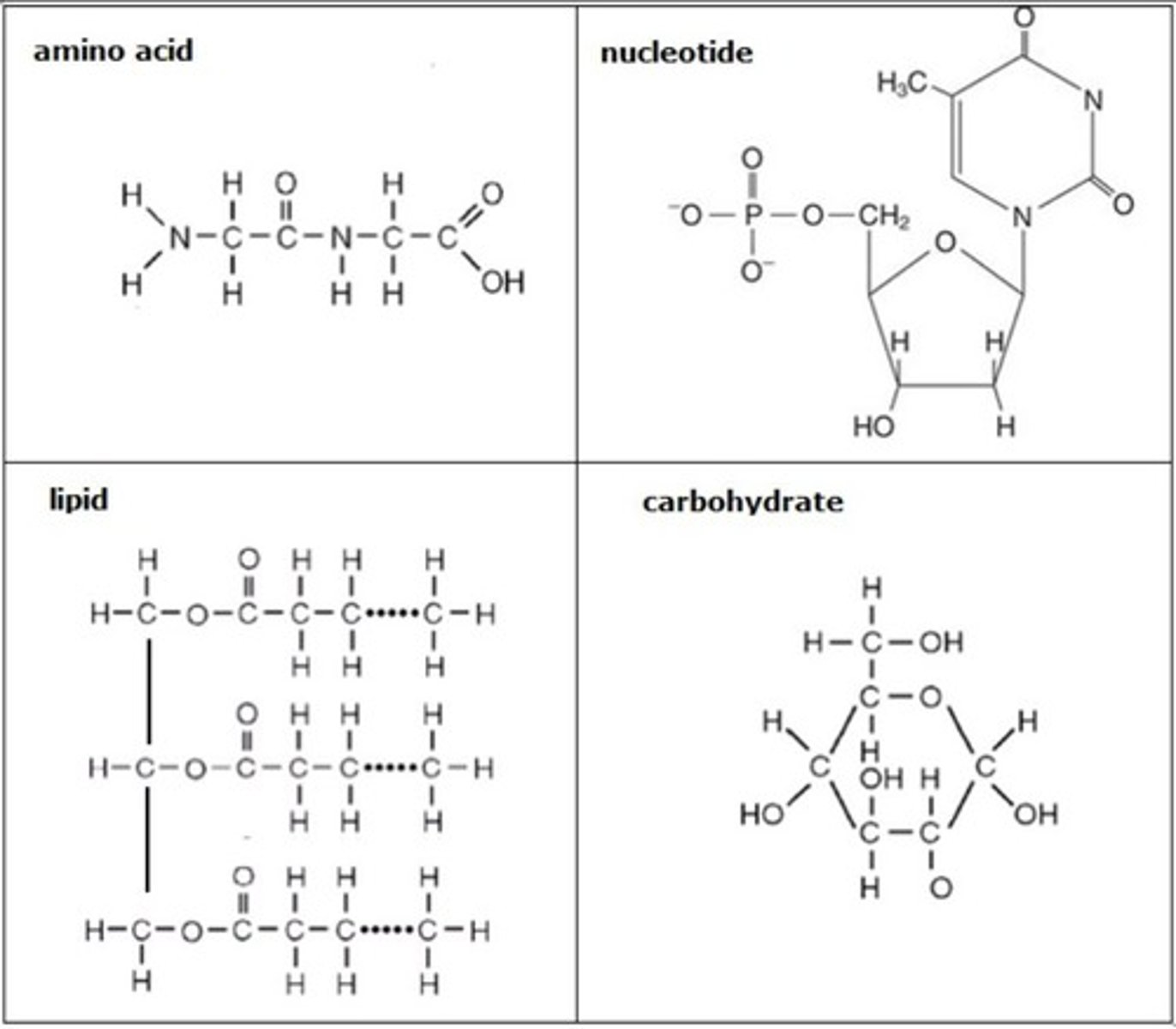
Organic molecules
Chemical compounds in which one or more atoms of carbon are covalently bonded to atoms of other elements.
Carbohydrates
A sugar molecule or polymer of sugar molecules used primarily for energy or to give structure to living organisms
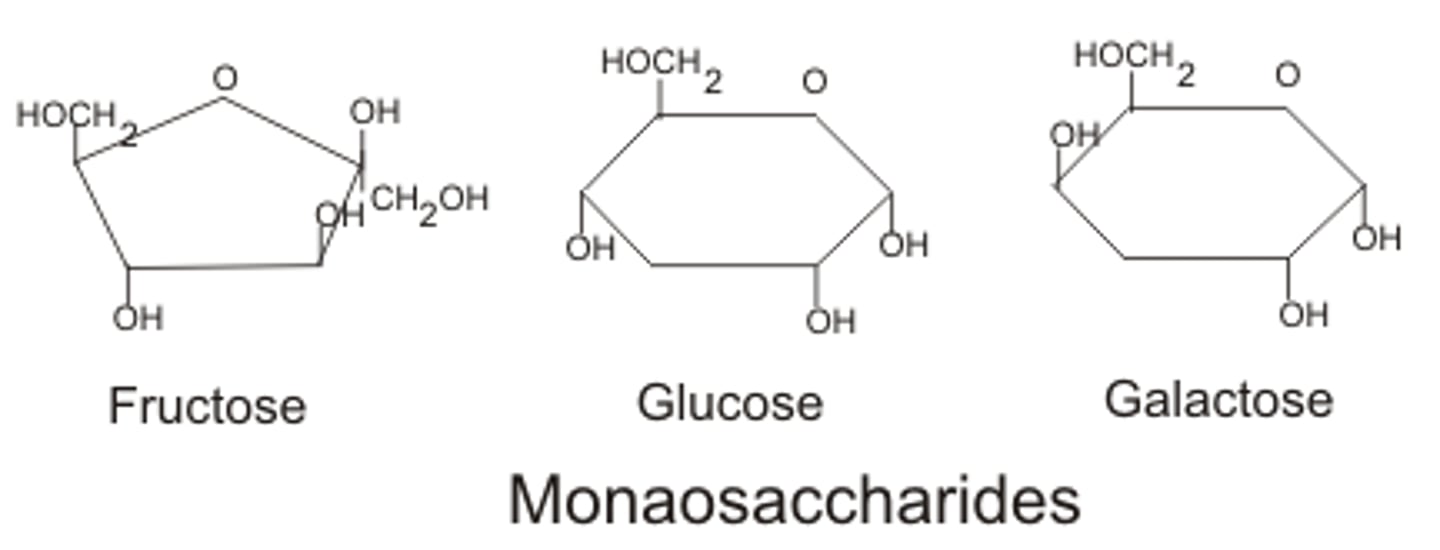
Monosaccharides
A single sugar unit or monomer. Examples include glucose, fructose, and galactose
Disaccharides
2 sugar monomers connected by a glycosidic linkage. Ex: sucrose (glucose + fructose) and lactose (glucose + galactose)
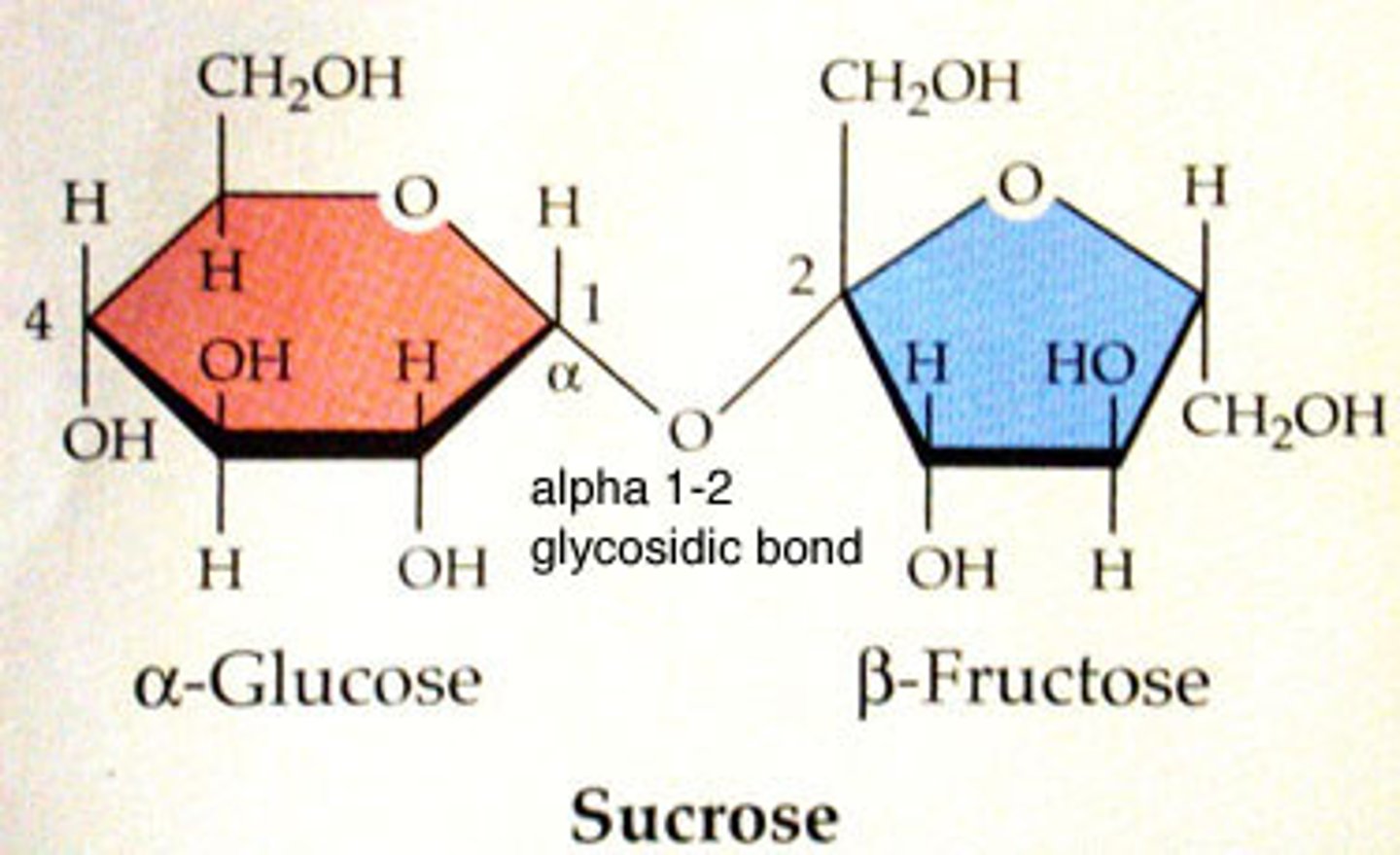
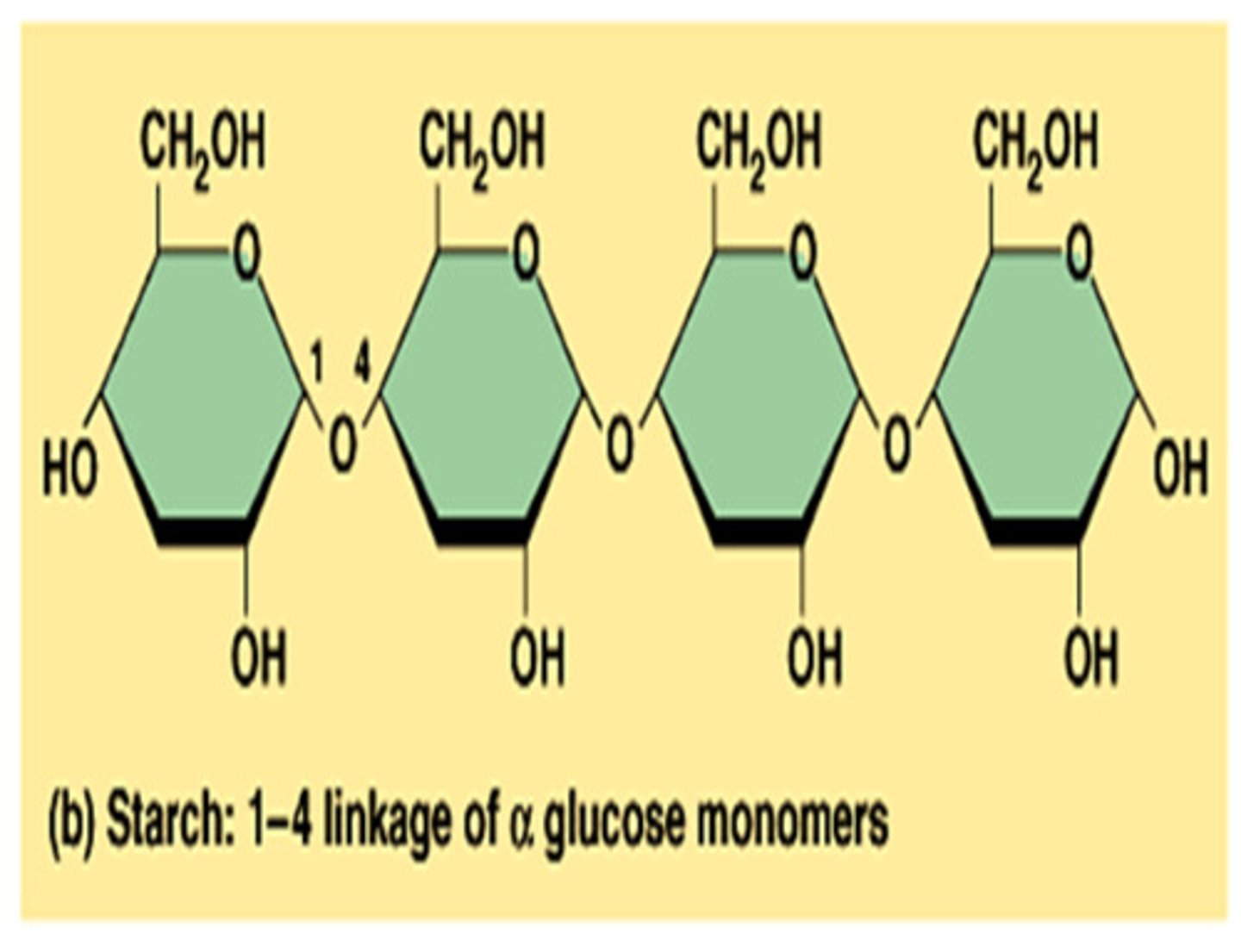
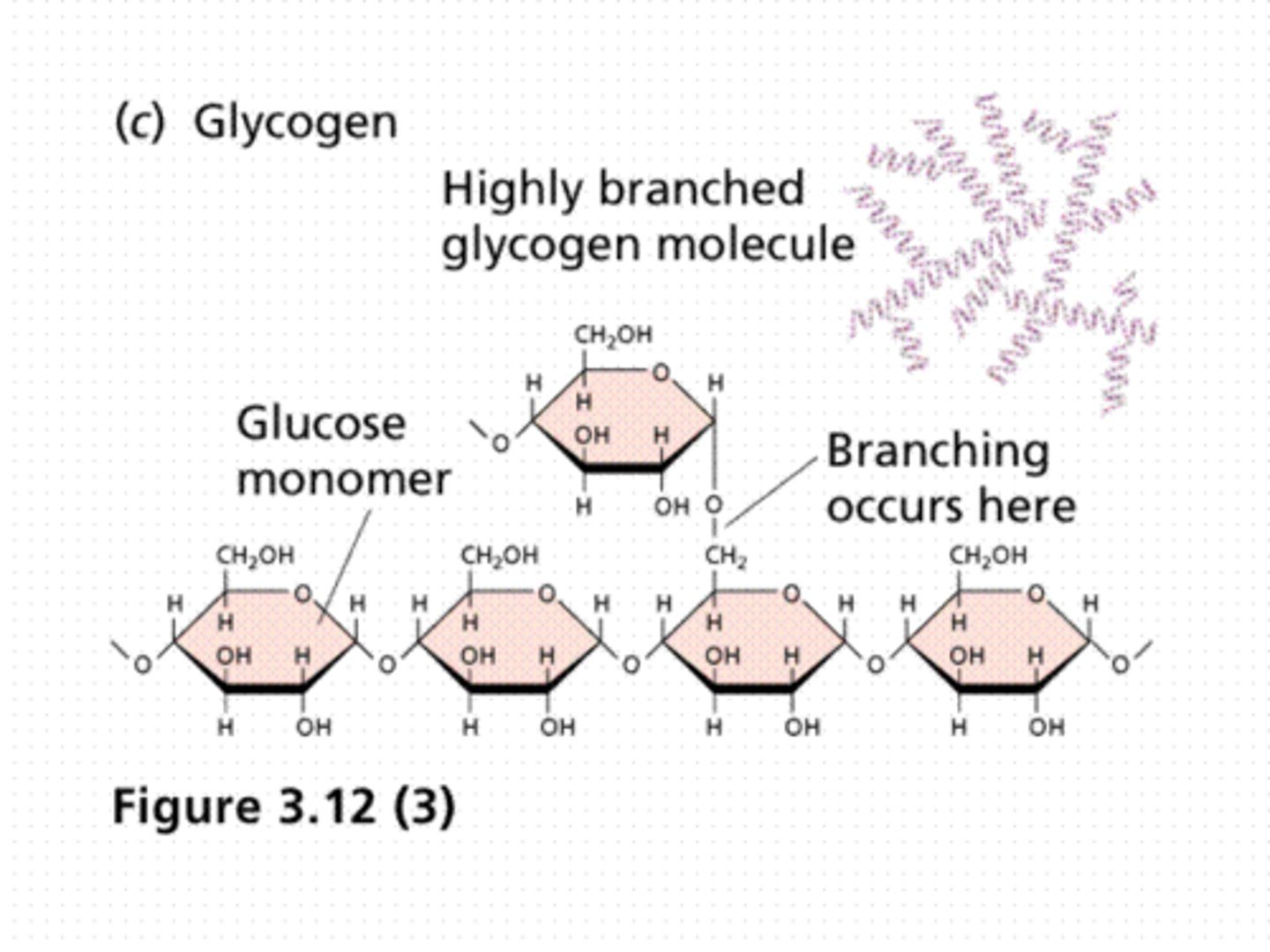
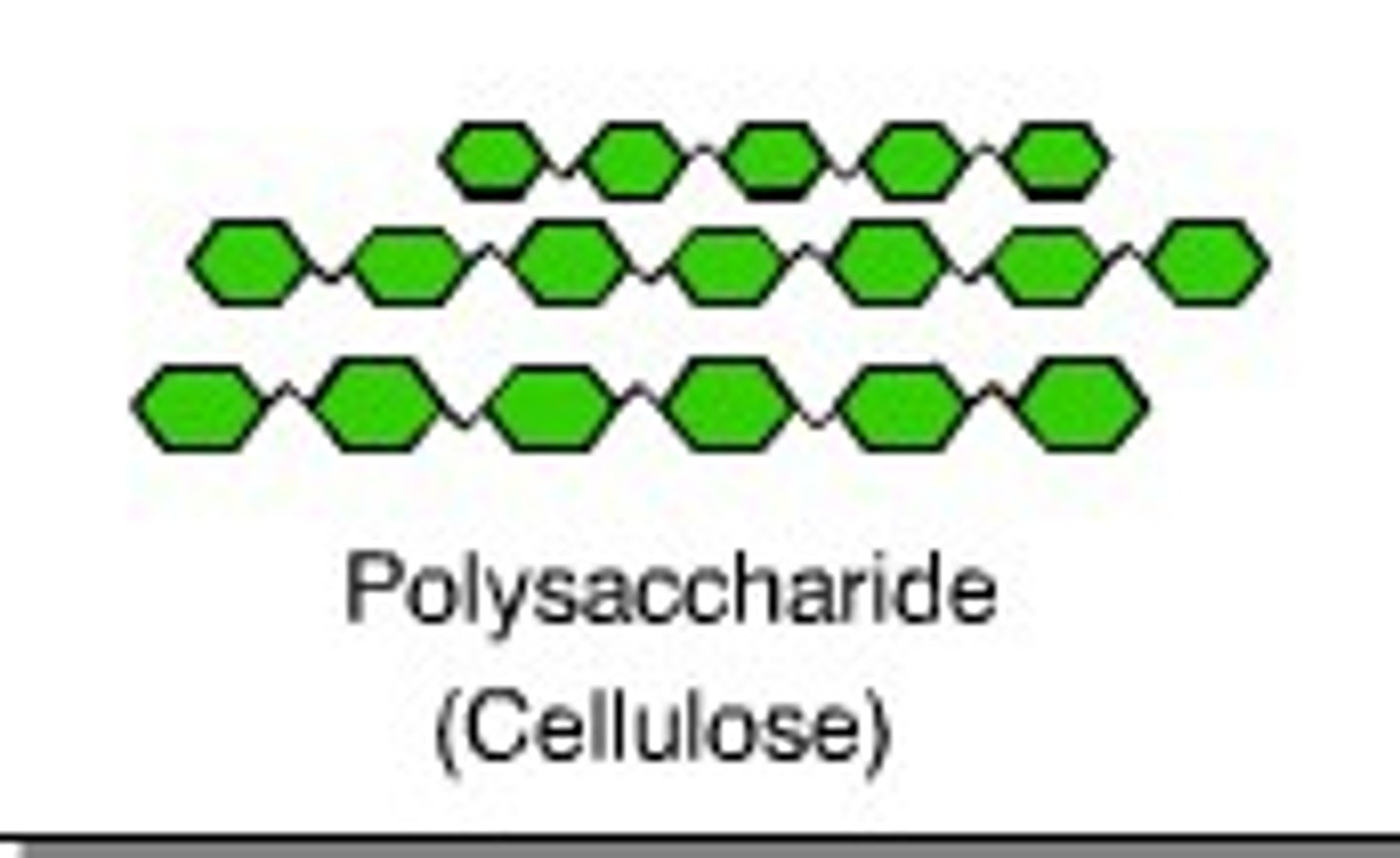
Polysccharides
Multiple sugar monomers connected by glycosidic linkages. Ex: glycogen (energy storage) and cellulose (structural support)
Glycogen
A polysaccharide formed from multiple glucose monomers that is used for energy storage in animals
Starch
A polysaccharide formed from multiple glucose monomers that is used for energy storage in plants.
Cellulose
A polysaccharide formed from multiple glucose monomers connected with a very sturdy glycosidic linkage that is used for structural support in plant cells.
Lipids
Biological molecules that are largely non polar and include fatty acids, steroids, and phospholipids. Mainly function in energy storage, insulation, cell signaling, and membrane structure.
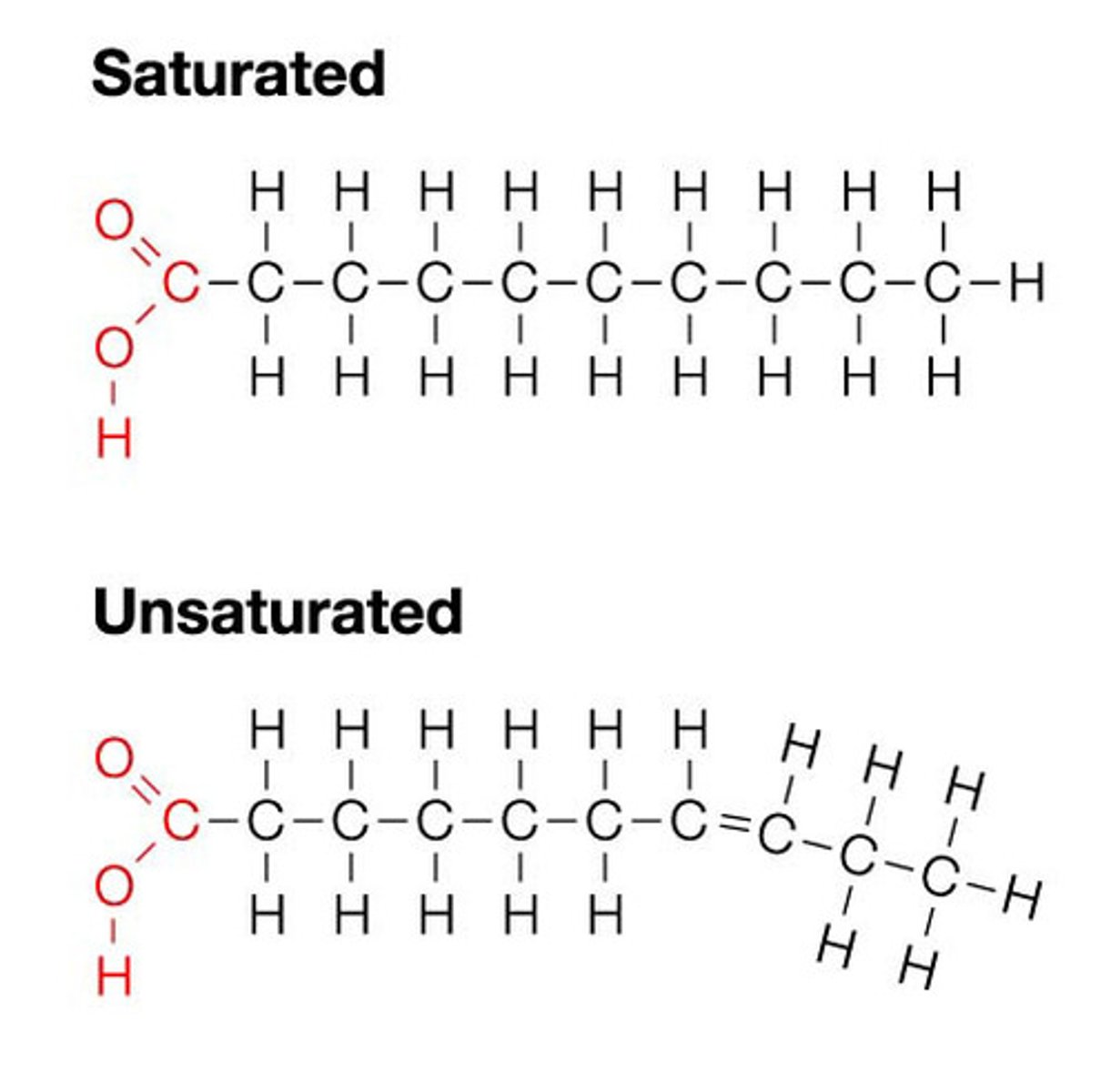
Fatty Acids
A lipid with a long carbon chain attached to a carboxylic acid group. They are nonpolar and are important in plasma membrane structure.
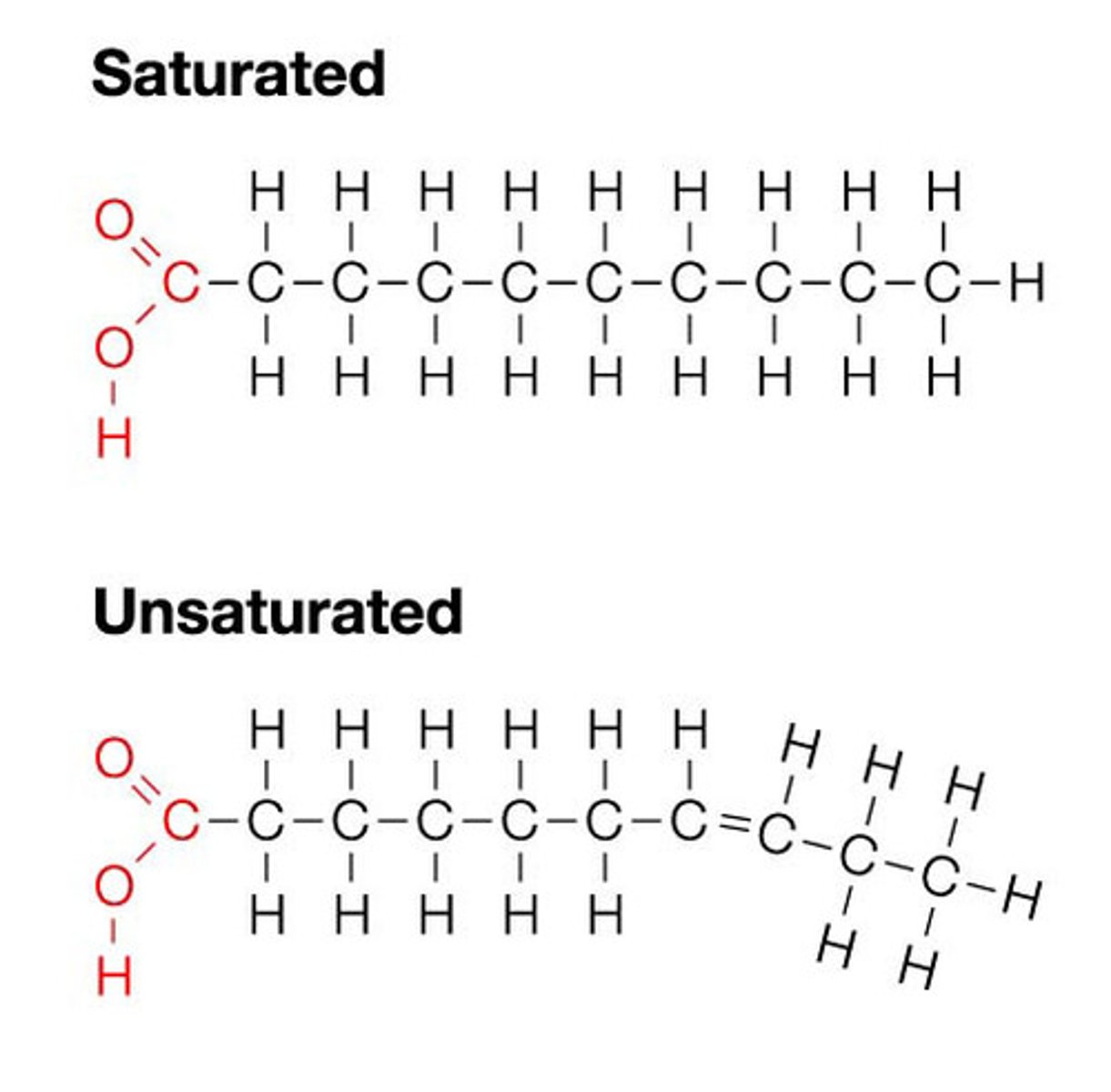
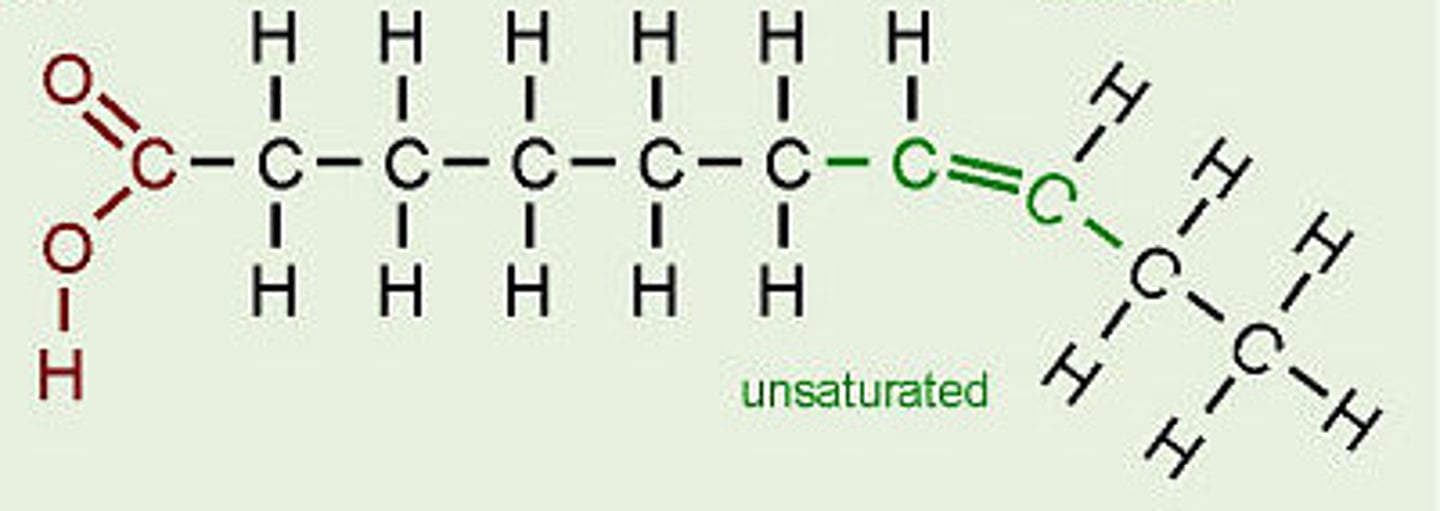
Unsaturated Fatty Acids
A fatty acid in which some of the carbons in the carbon chain are connected by double bonds. They are typically liquid at room temp and give flexibility to membranes.
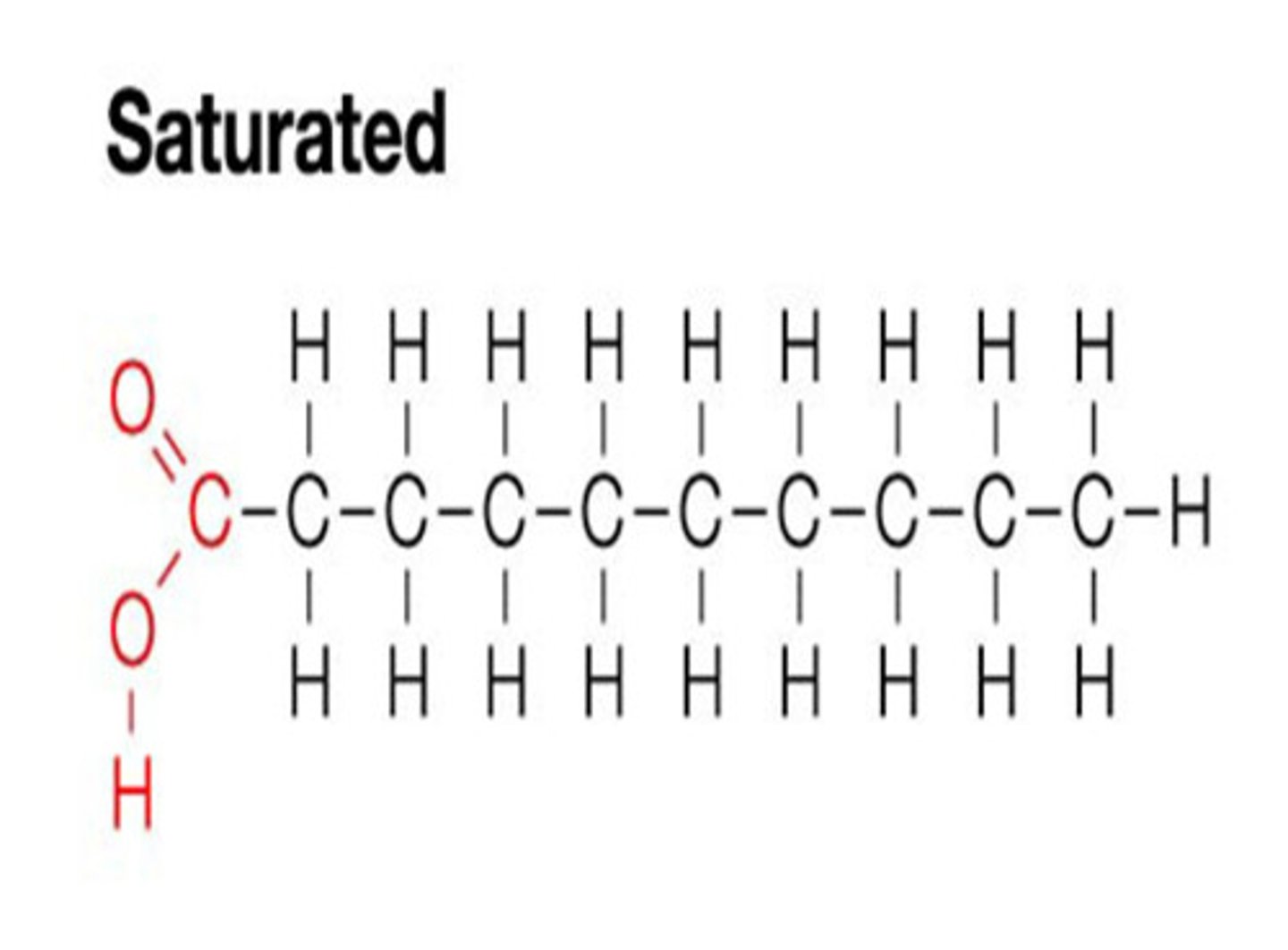
Saturated Fatty Acids
A fatty acid in which all of the carbons in the carbon chain are connected with single bonds. These are typically solid at room temperature and give rigidity to membranes.
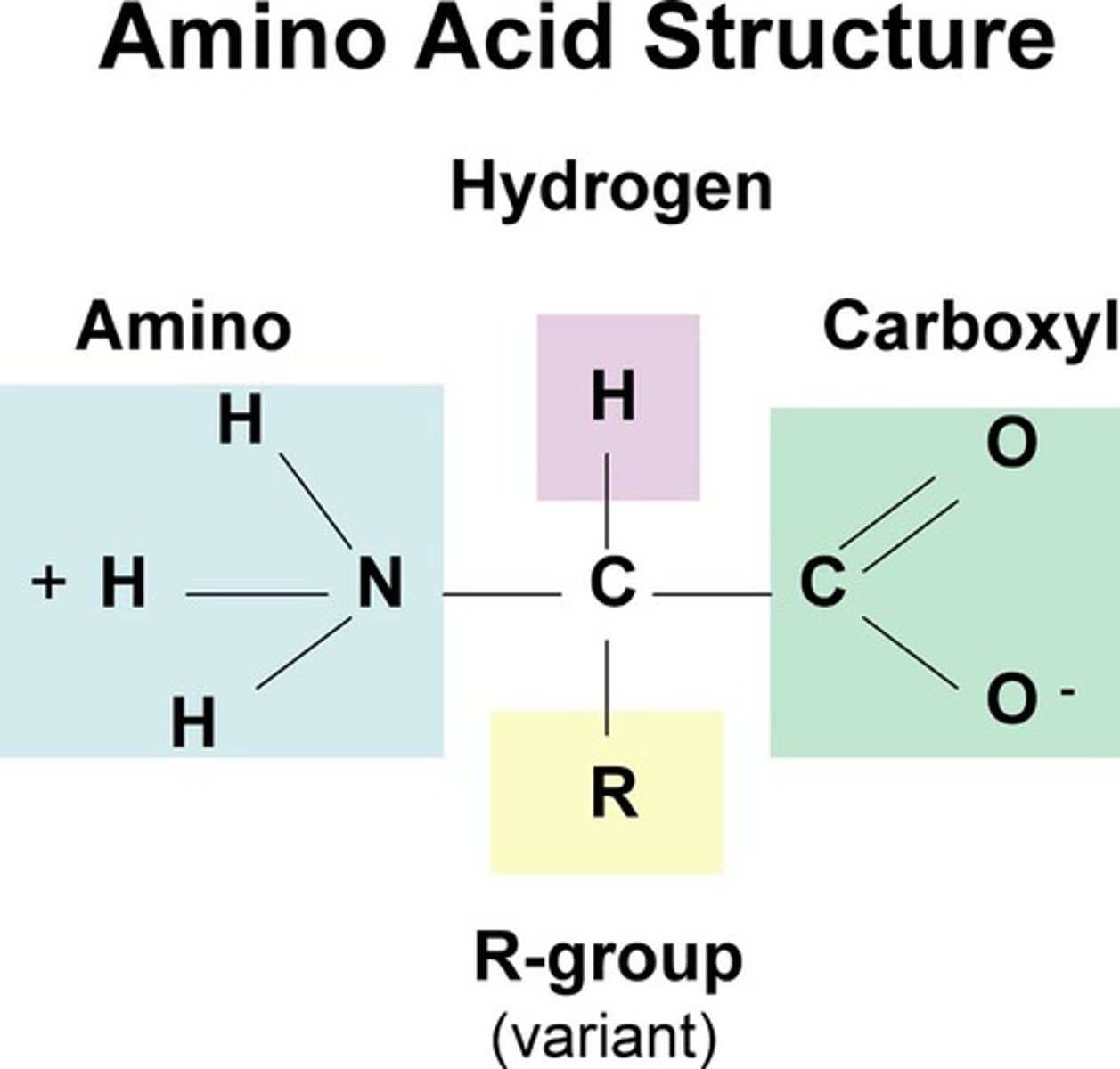
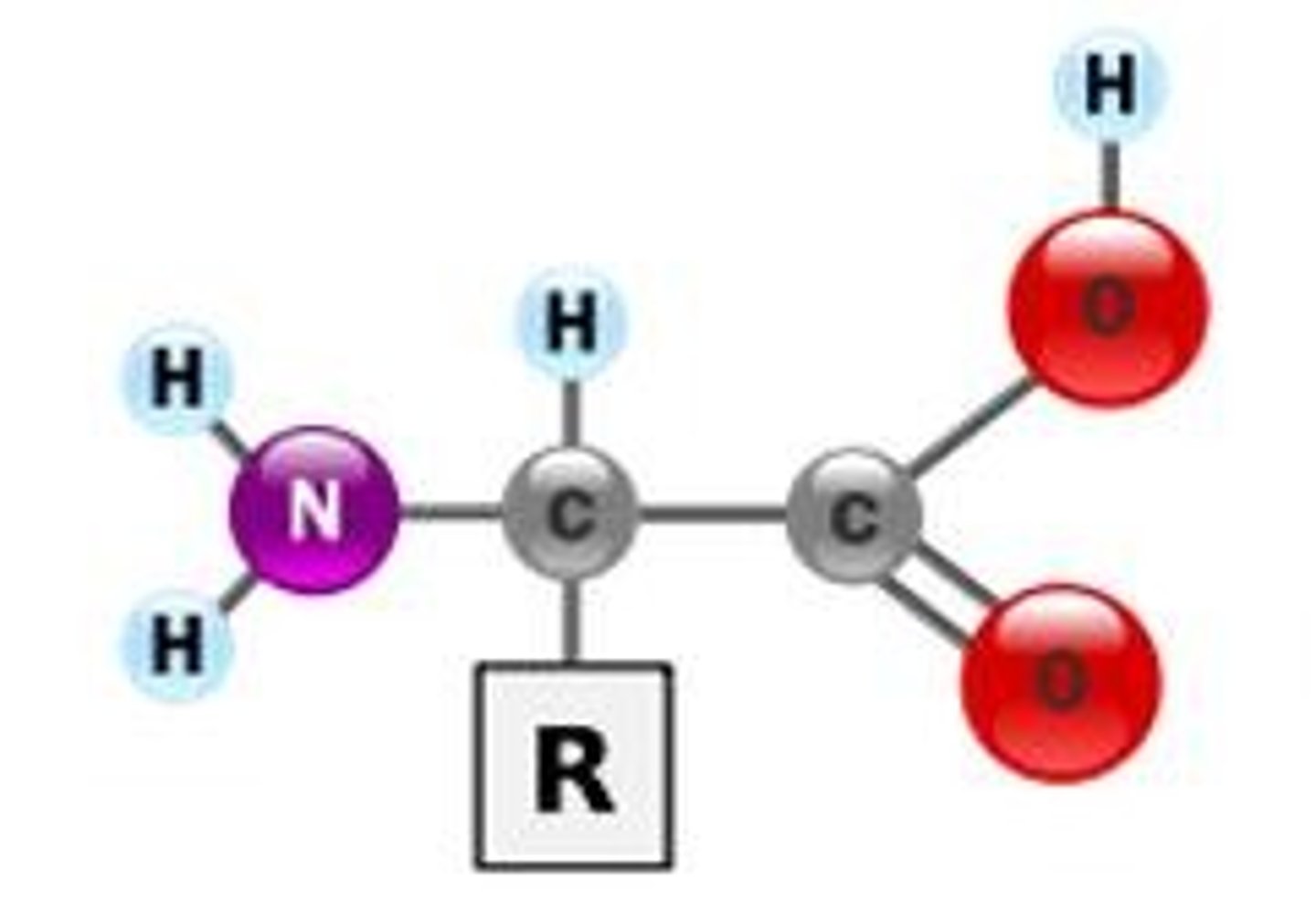
Amino Acid
The building block or monomer from which proteins are made. They consist of a central carbon which a carboxyl group, amino group, and an R-group. The R-group determines the identities and properties of this molecule.
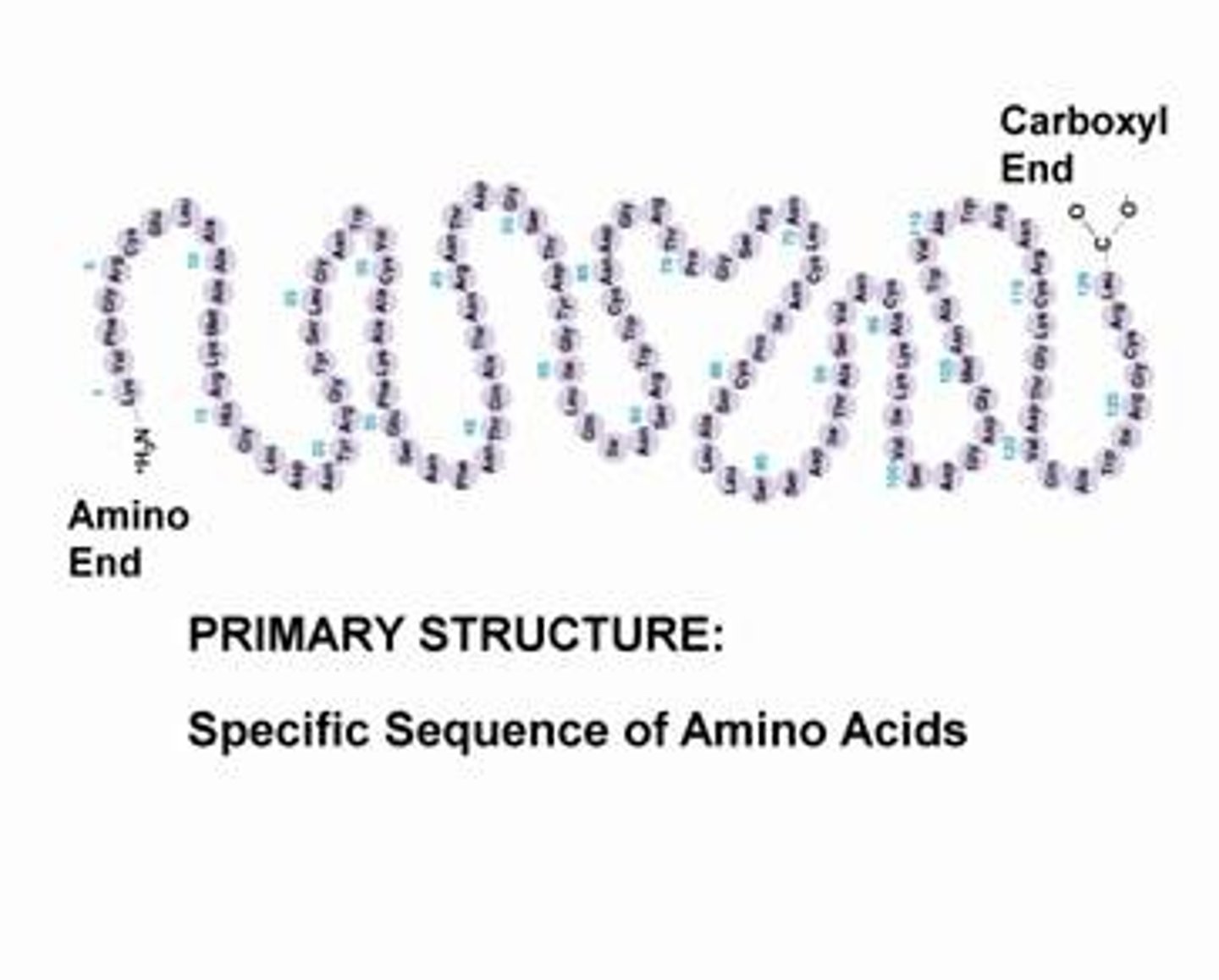
Primary structure
sequence of amino acids in a polypeptide chain. It is formed by peptide bonds between the amino acids.
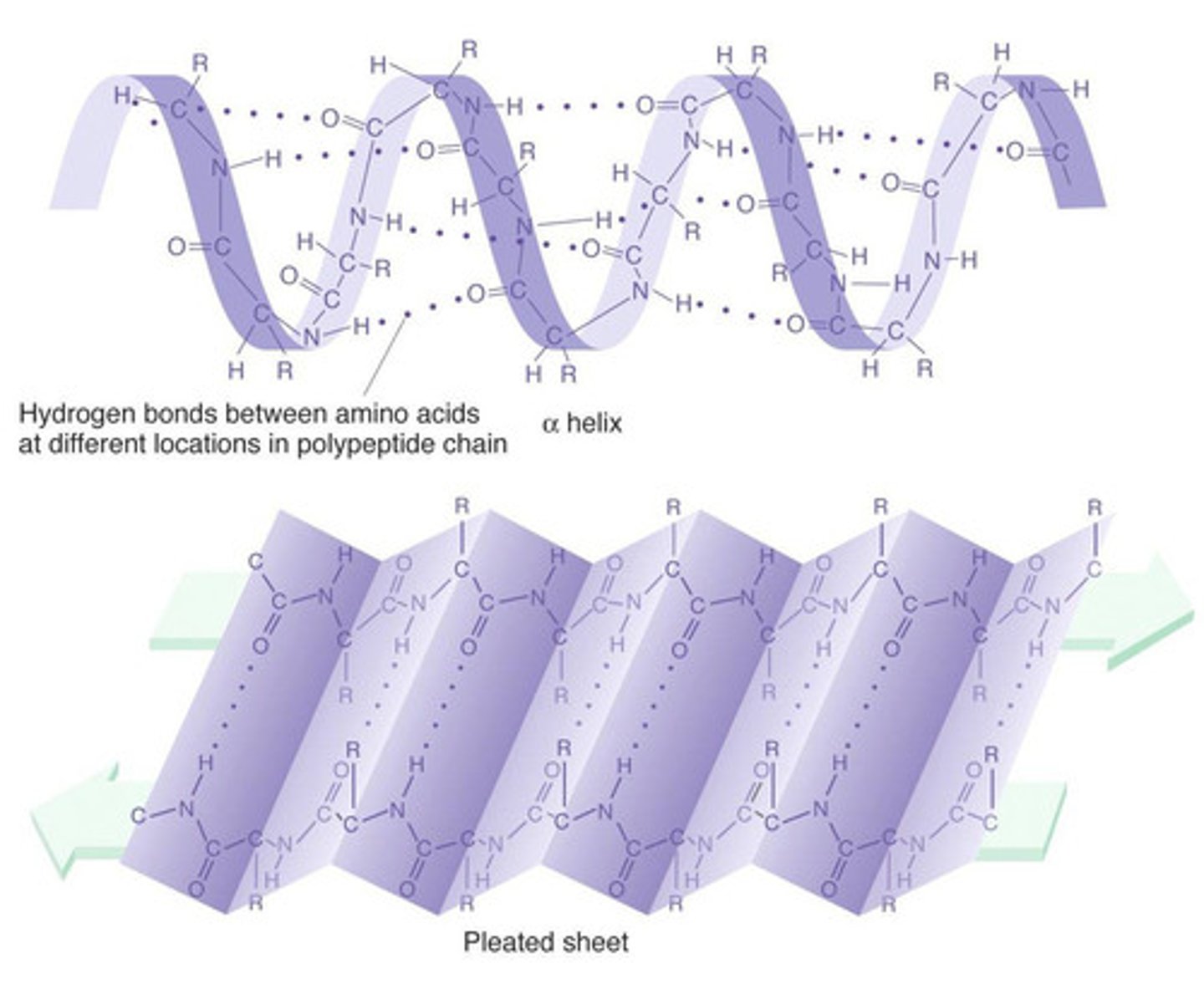
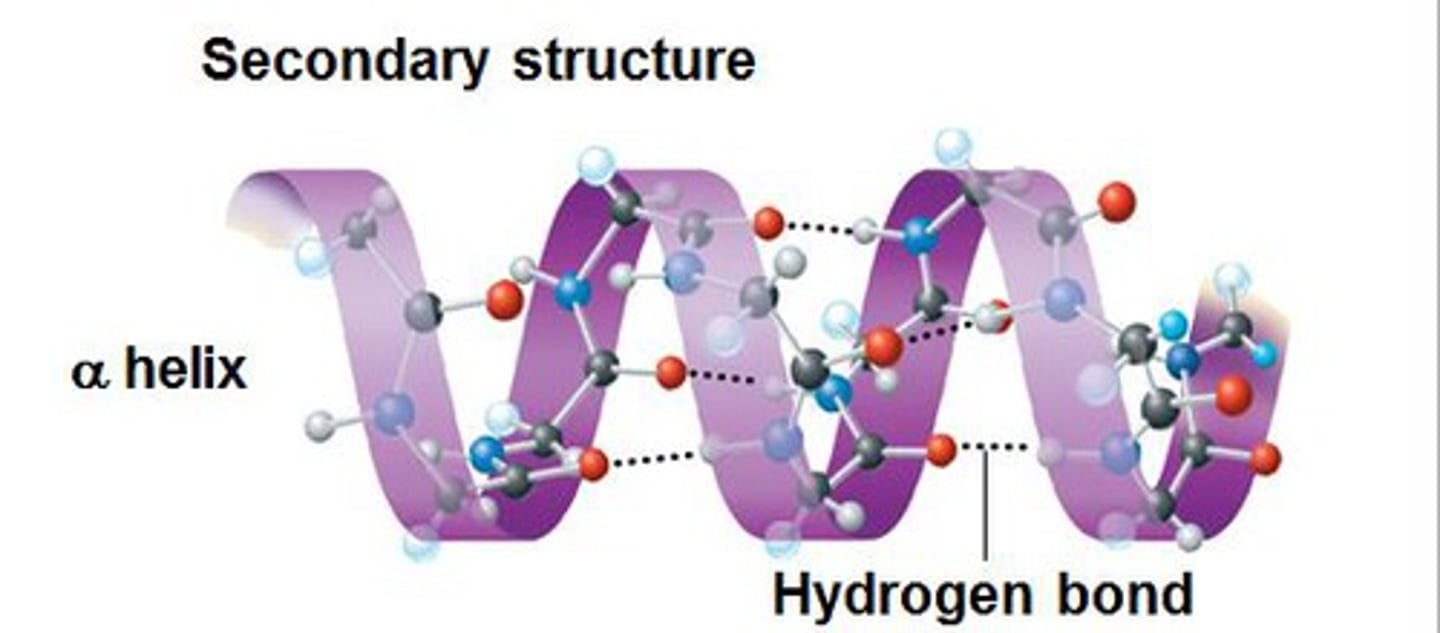
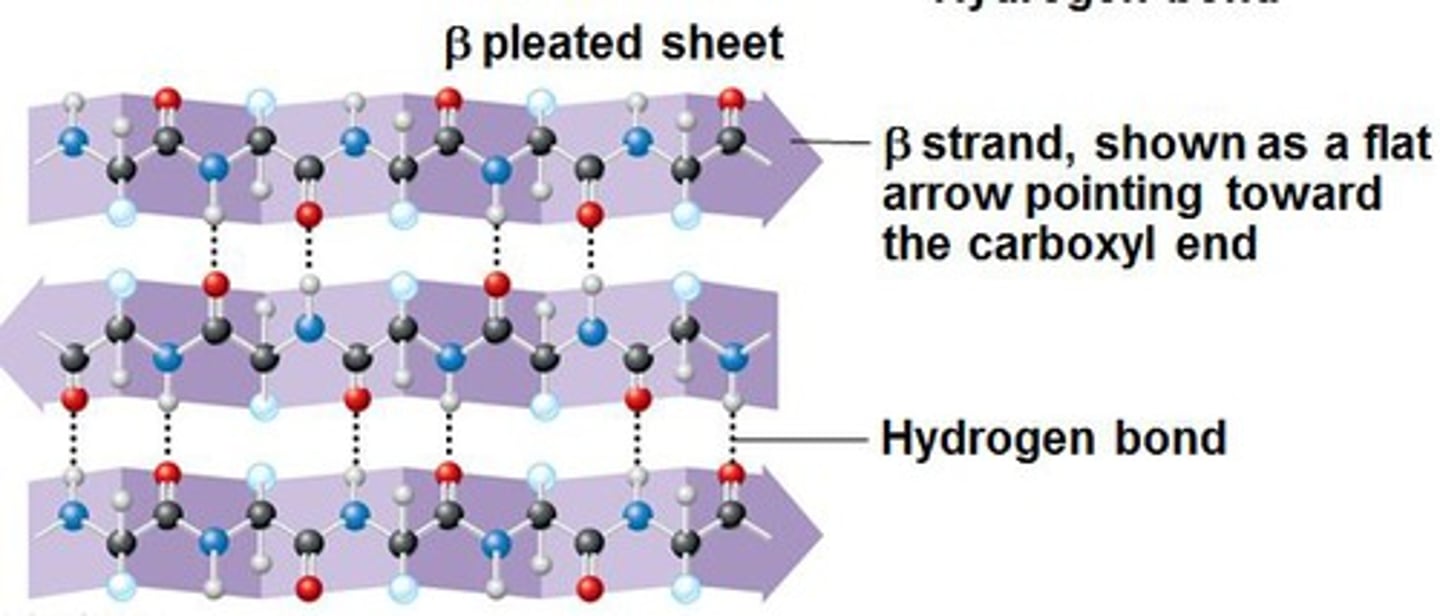
Secondary Structure
The arrangement of amino acids in a polypeptide chain driven by hydrogen bonding between the amino acids in the polypeptide chain. Two forms: alpha helices and beta sheets.
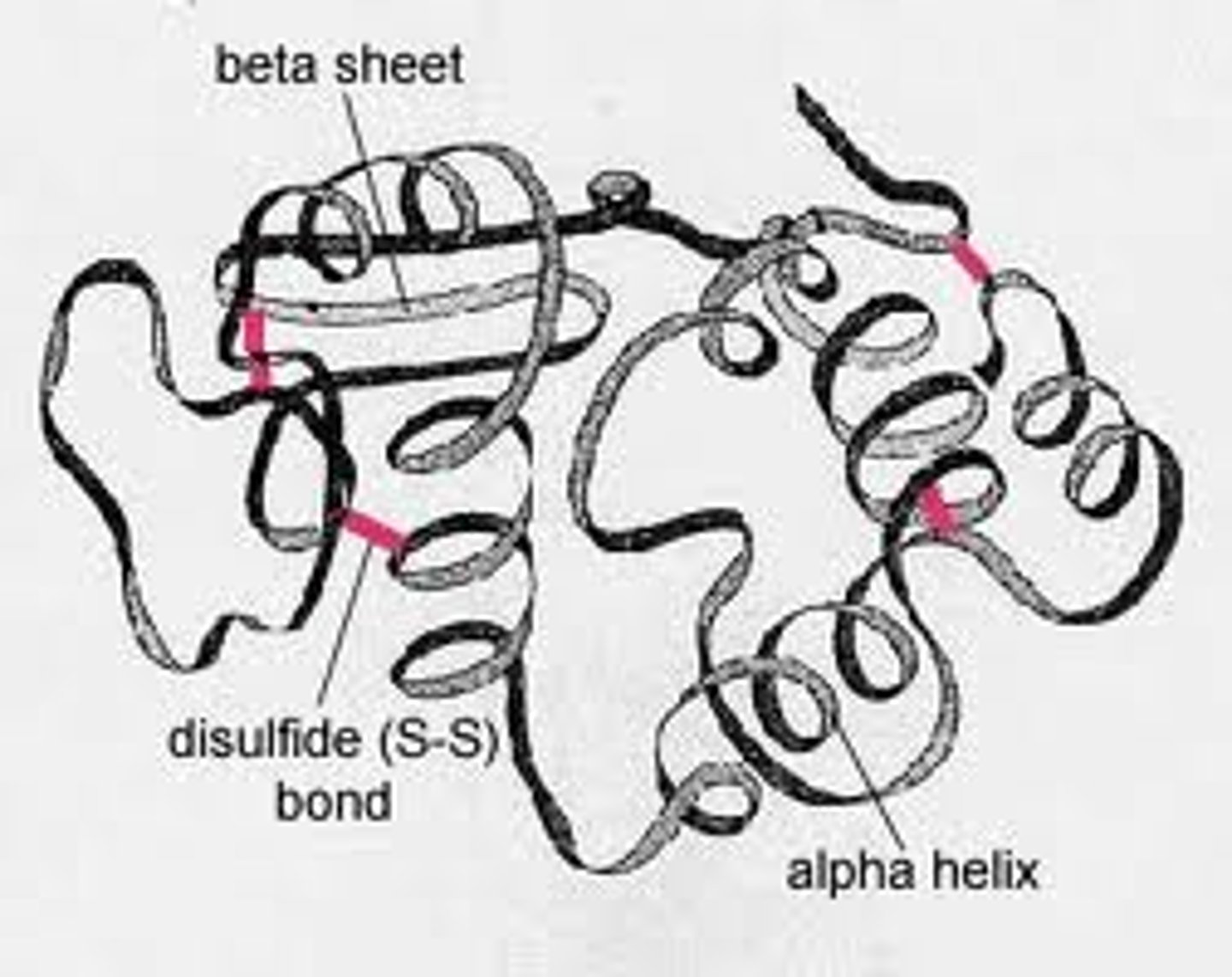
Tertiary Structure
Driven by interactions between R-groups in a polypeptide chain and can include hydrophobic interactions, hydrogen bonding, and disulfide bridges.
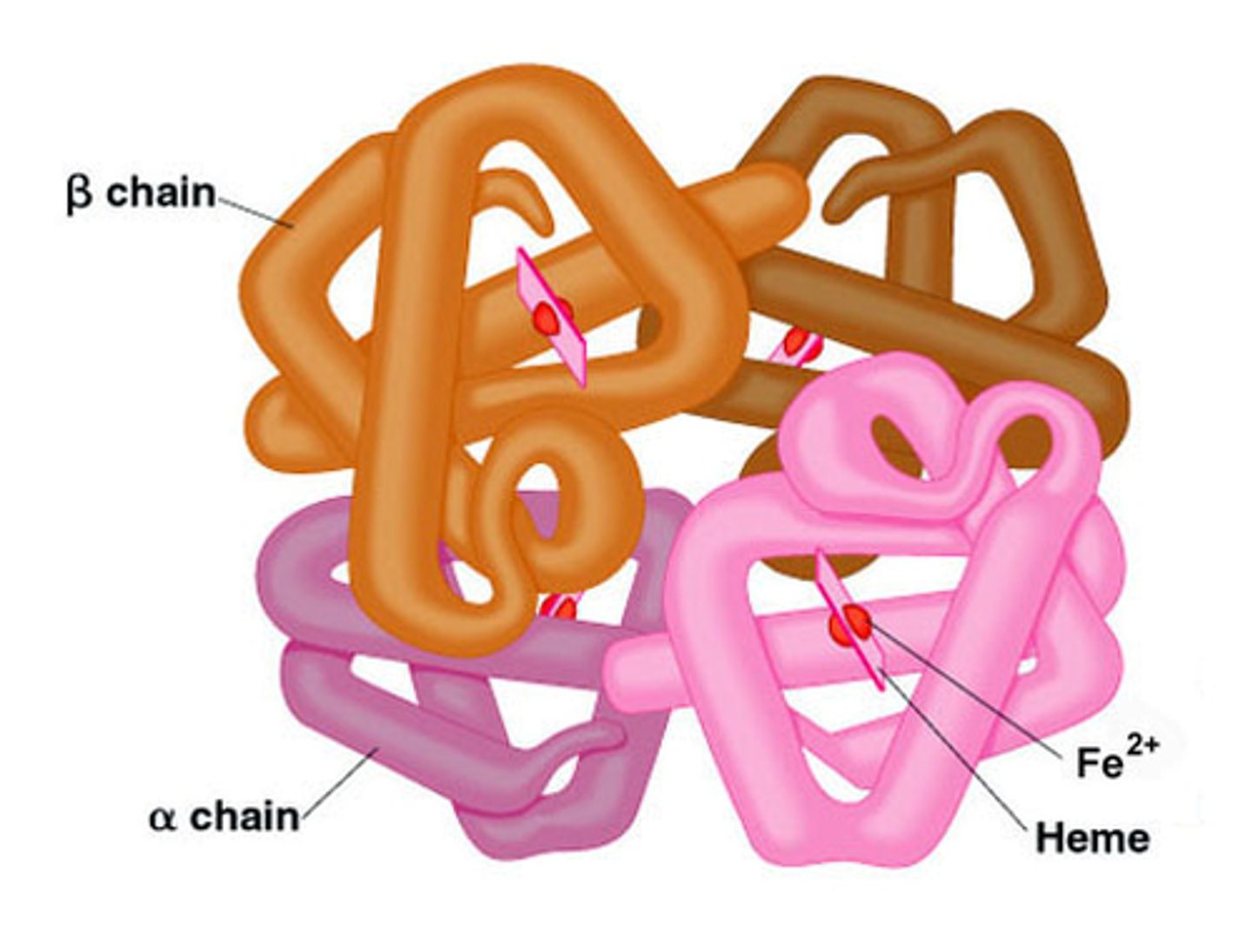
Quaternary Structure
Found only in proteins composed of more than one subunit (hemoglobin and collagen)
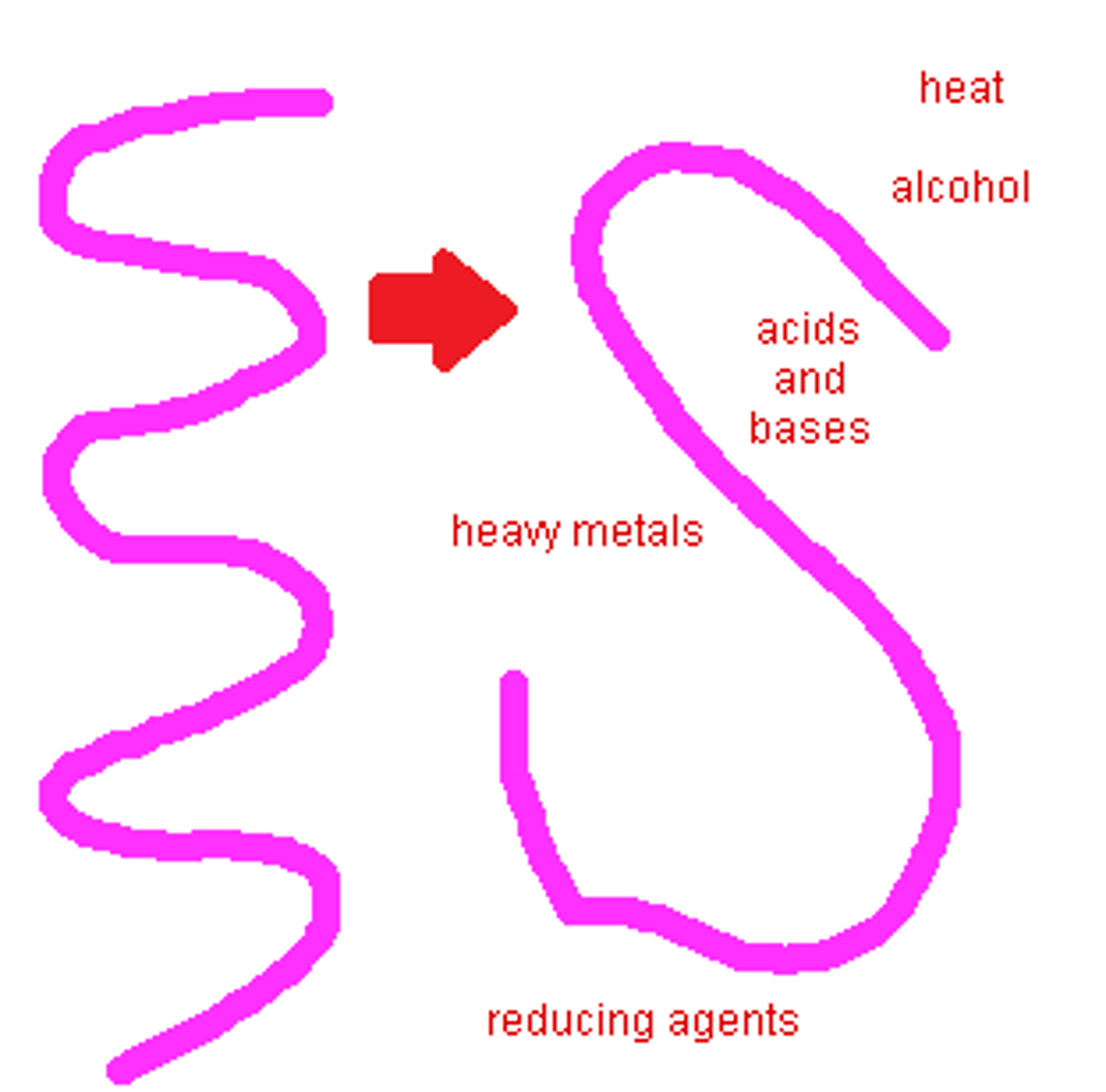
Denatured
Describes a protein that has changed its shape and does not function as usual. This may be caused by changes in temperature, pH, or salt concentration
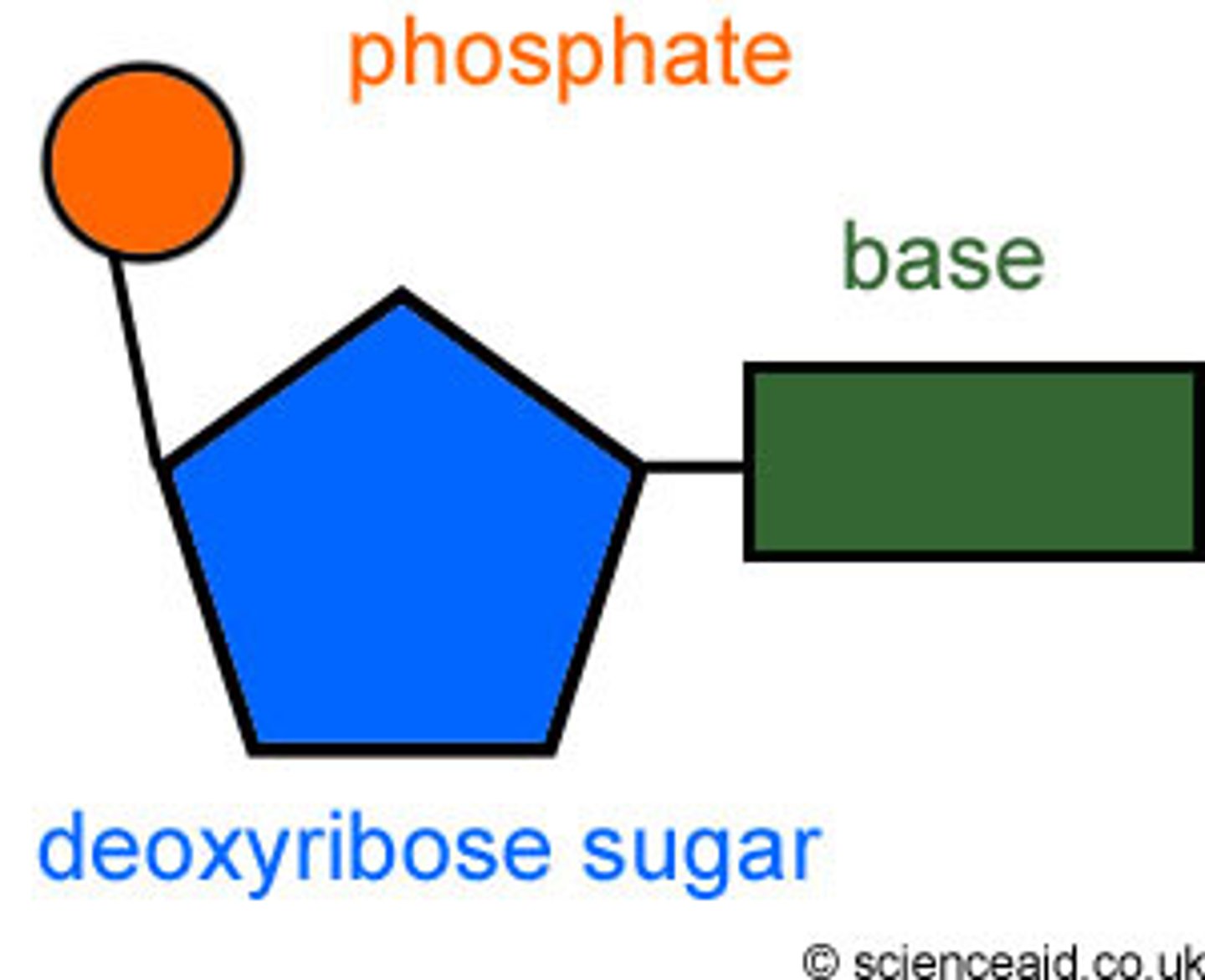
Nucleic Acids
Molecules that can store/transmit genetic info and contain a sugar-phosphate backbone and nitrogenous bases.
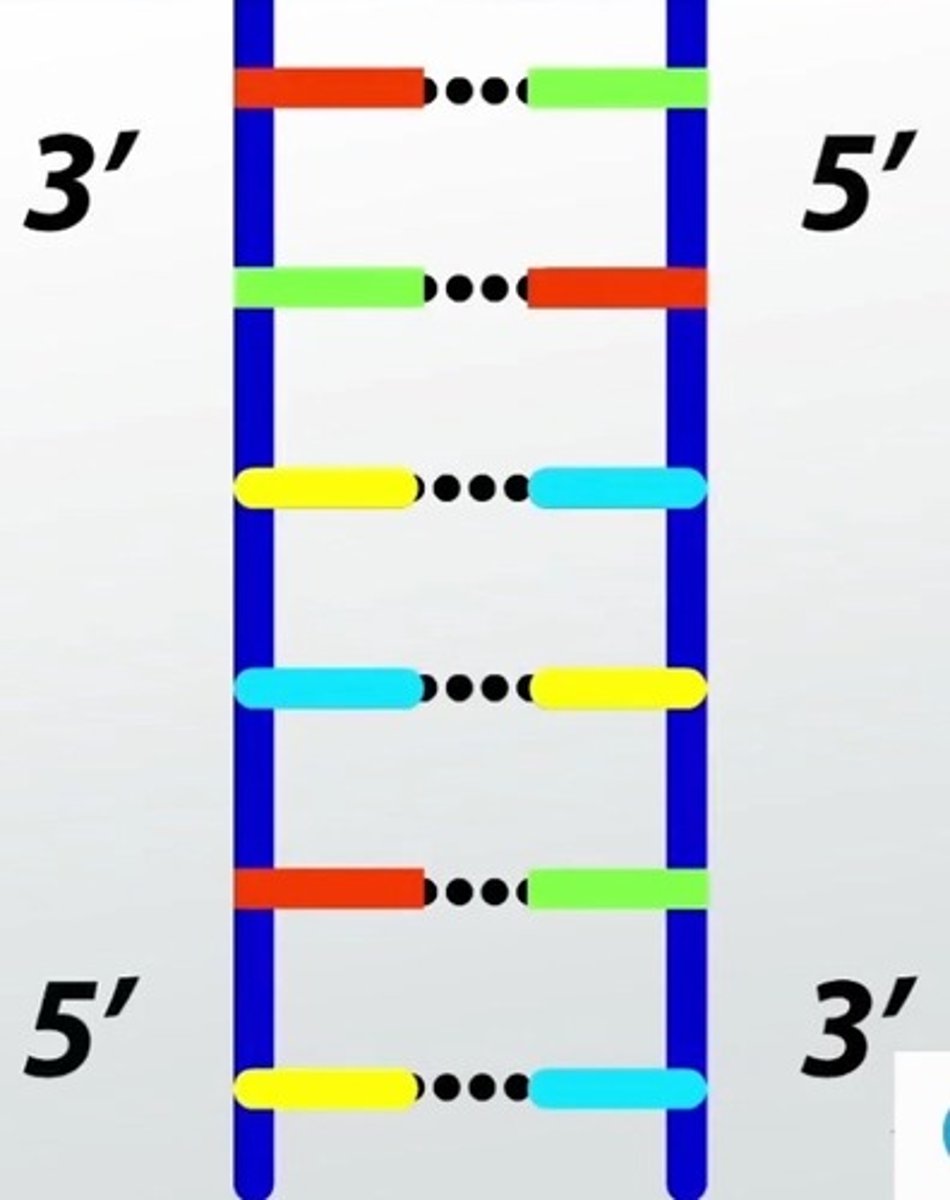
Antiparrallel
Describes the orientation of the 2 strands of the DNA double helix. One strand of the DNA double helix starts with a 5' phosphate group. The complementary strand would start with a 3' hydroxyl group.
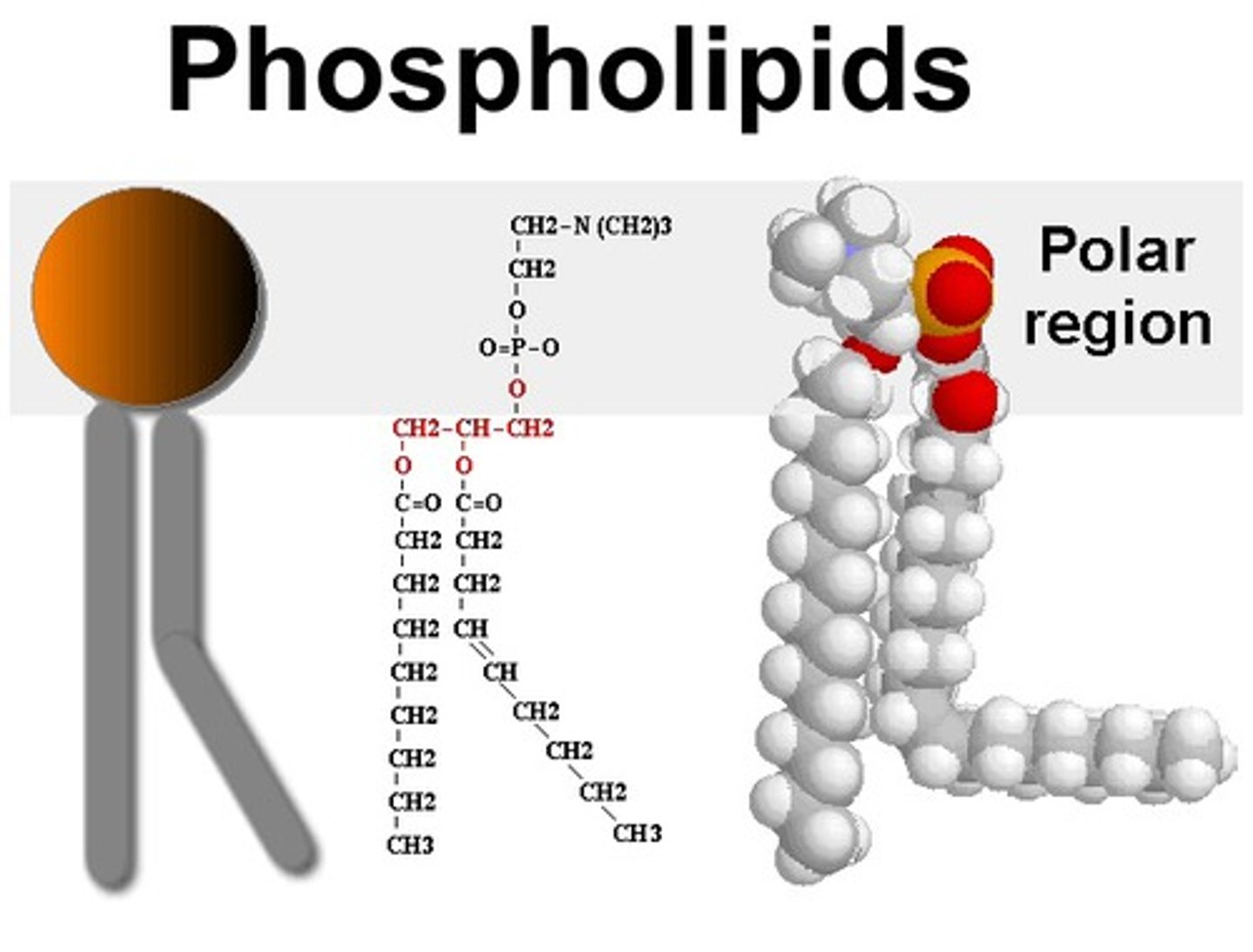
Amphipathic
Describes molecules, such as phospholipids, that have both polar and nonpolar components.
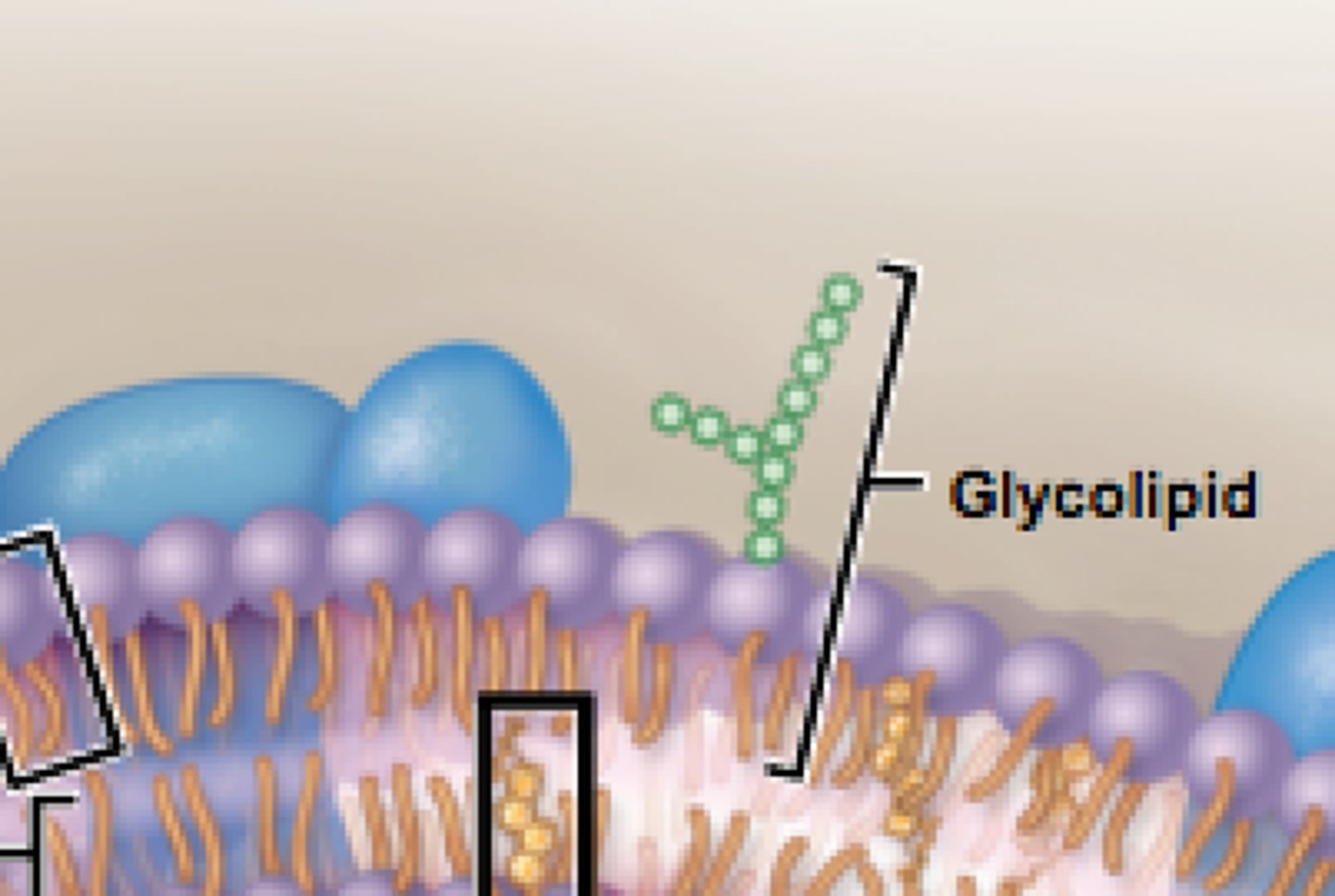
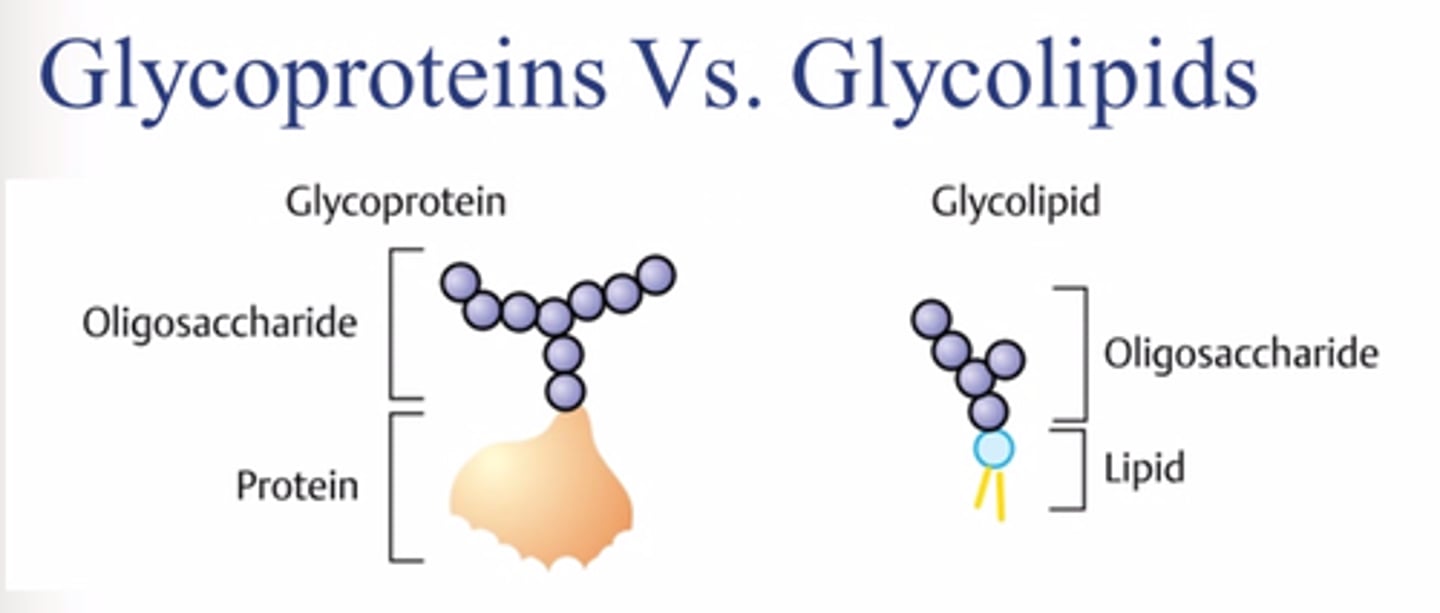
Glycolipids
Lipids with added carbohydrate groups. In cell membranes, these function in cell recognition.
Glycoproteins
Proteins with added carbohydrate groups. In cell membranes, these function in cell recognition.
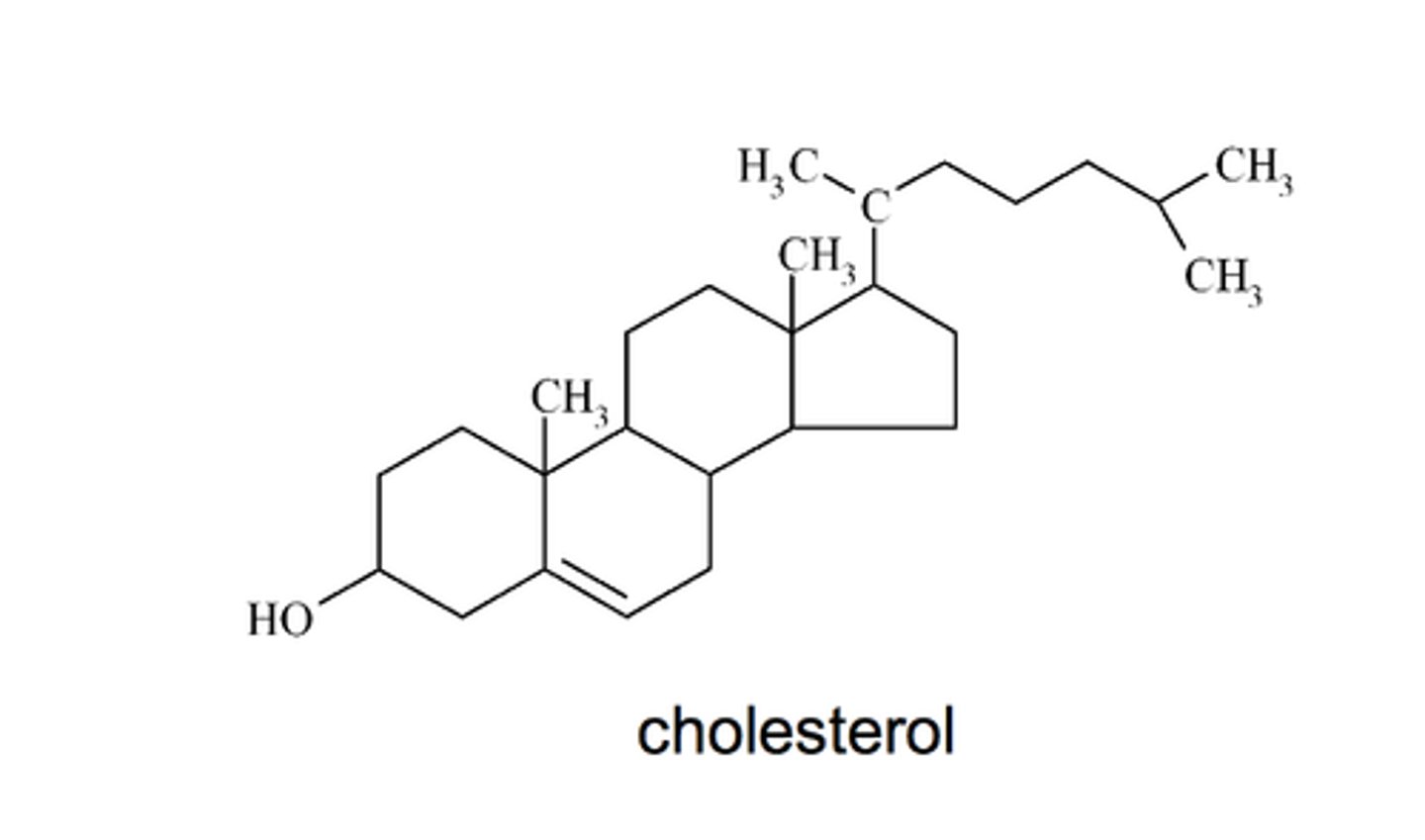
Steroids
Lipids that have a flat structure made of four fused carbon rings with additional functional groups attached. They can function as ligands in cell signaling. One example is cholesterol, which helps to regulate the flexibility of cell membranes.
Proteins
Biological molecules that are made of chains of amino acids joined by peptide bonds. They have many functions including molecule transport, reception of signals, enzyme catalysis, and intercellular joining.
Alpha helices
The coiled structure of a polypeptide chain that is held together by hydrogen bonds between the carboxyl and amino groups within a polypeptide chain. They are part of the secondary protein structure.
Beta Sheets
A type of secondary structure found in proteins in which two or more parallel and adjacent polypeptide chains are arranged so that hydrogen bonds can form between the carboxyl and amino groups on different chains.
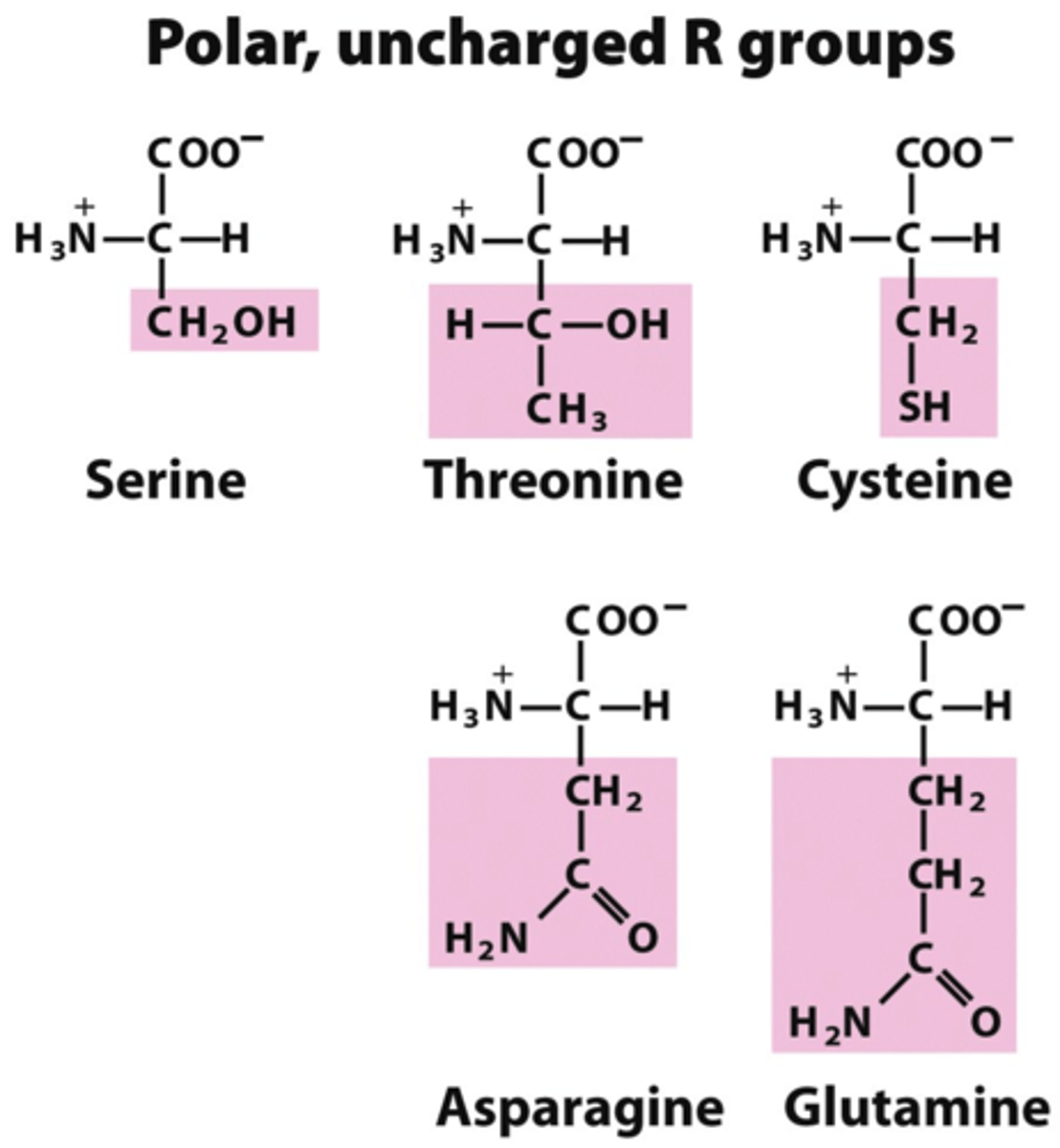
R-groups
Gives amino acids their specific properties. They can be polar or nonpolar and can be uncharged or carry positive or negative charges. These groups determine the identity of the amino acid.
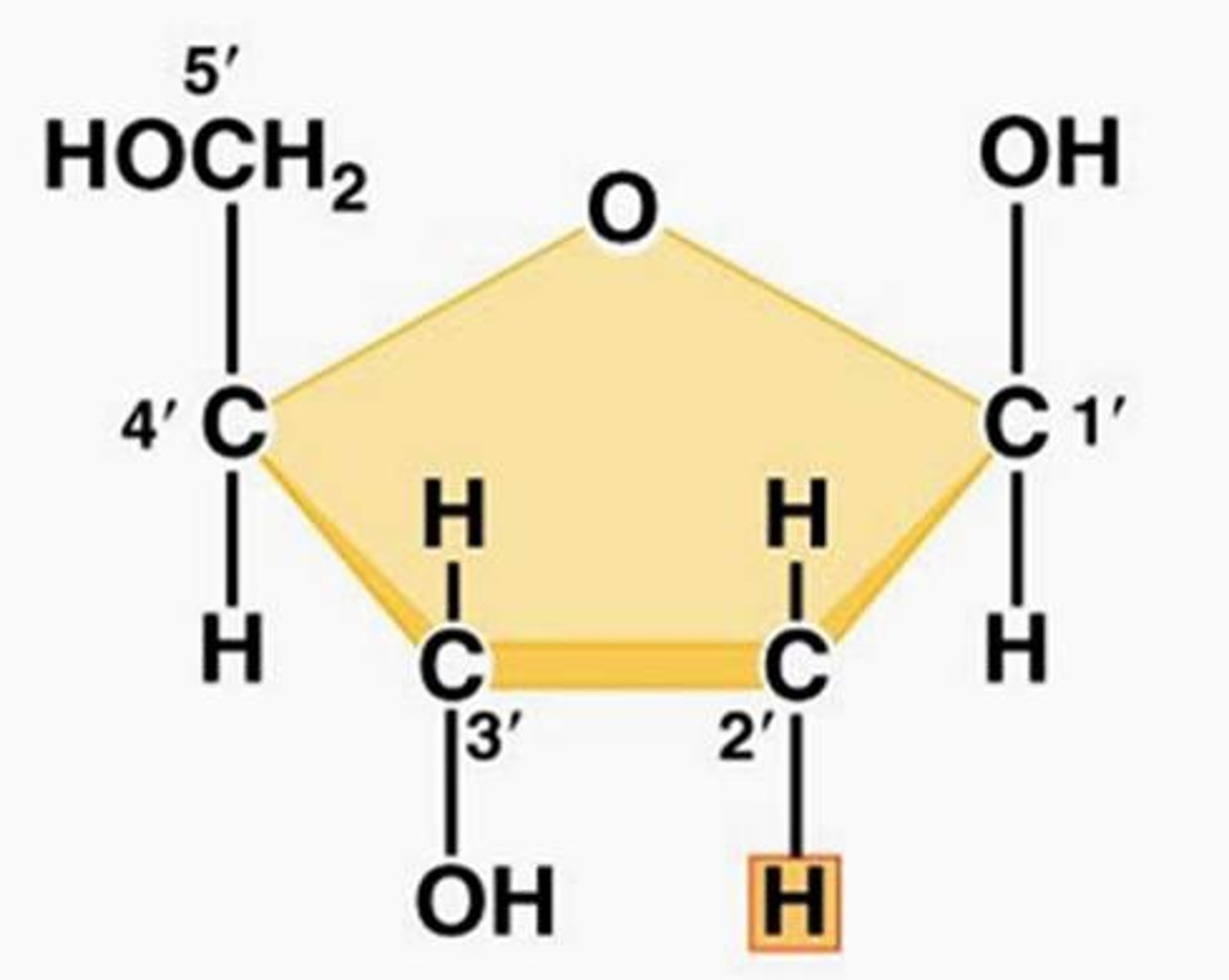
Deoxyribose
A five-carbon sugar that is a component of DNA nucleotides. It doesn't contain a hydroxyl on its 2' carbon and thus is more stable than ribose.
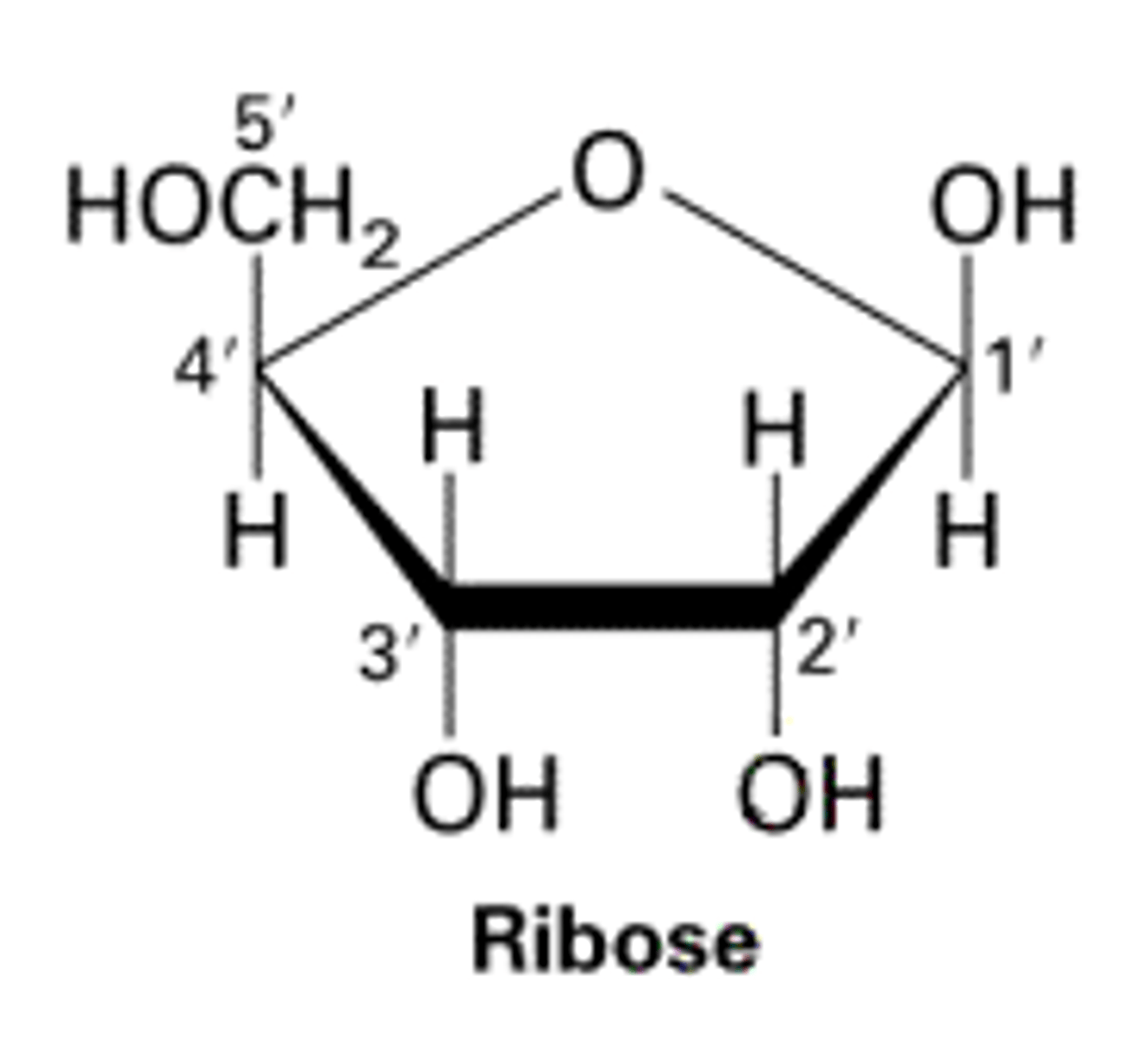
Ribose
A five-carbon sugar found in RNA. It is less stable than deoxyribose.
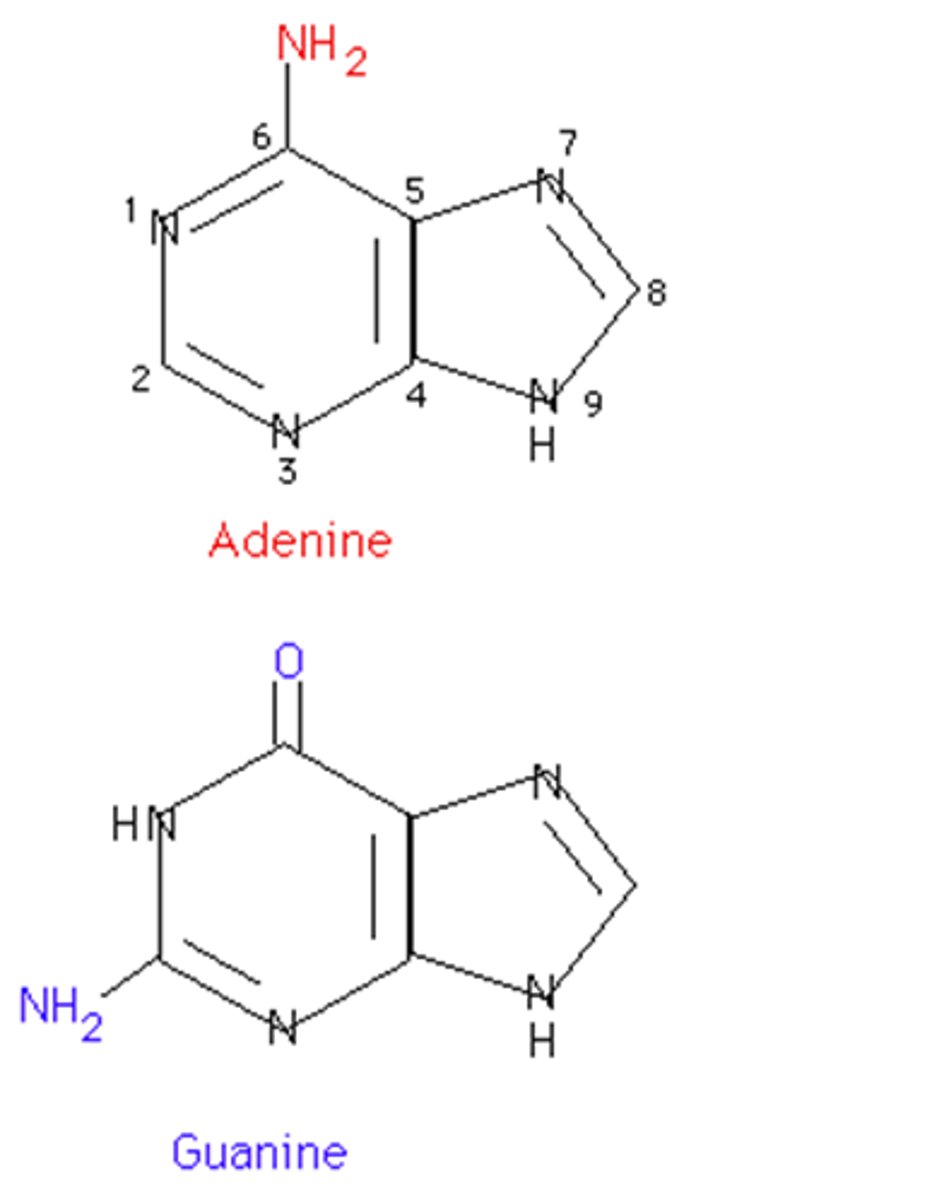
Purines
two fused hydrocarbon rings. Ex: adenine and guanine
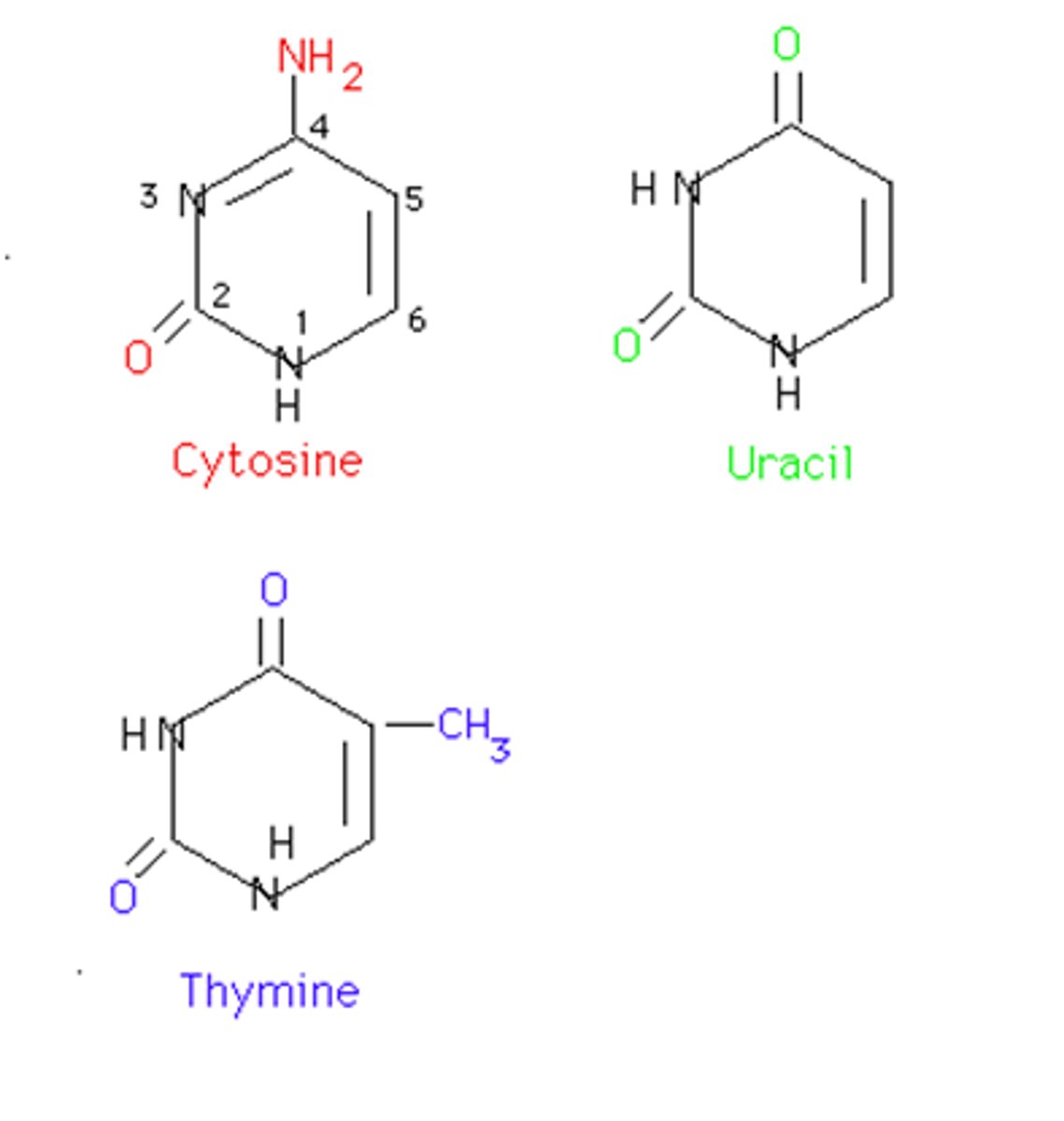
Pyrimidines
single hydrocarbon rings. Ex: thymine, cytosine, and uracil