Titration Curves
0.0(0)
0.0(0)
Card Sorting
1/7
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
New cards
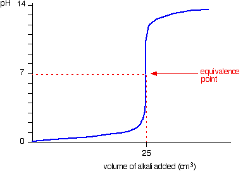
Strong Base into Strong Acid (strong acid titrated by a strong base)
2
New cards
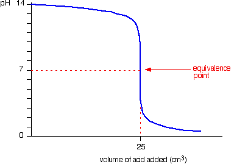
Strong Acid into Strong Base (or a strong base titrated by a strong acid)
3
New cards
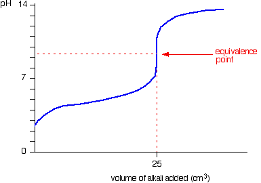
Strong Base into weak acid
4
New cards
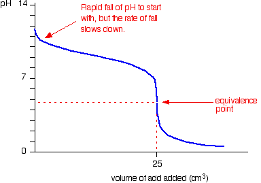
Strong Acid into Weak Base
5
New cards
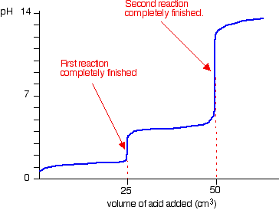
Strong Base into Weak Diprotic Acid
6
New cards
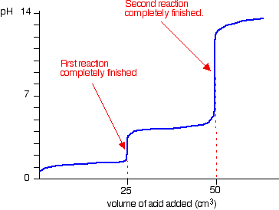
Strong Acid into Weak Diprotic base Species
7
New cards
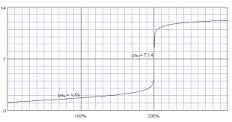
Why is sulfuric acid an exception?
Because it is the only diprotic strong acid, so it only has 1 equivalence point
8
New cards
What is the blood pH buffer pair?
Human blood contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO−3) in order to maintain blood pH between 7.35 and 7.45