1.3C Electron Configurations of Atoms and Ions
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
Why do we use Noble Gases as shorthands? What are the electron configurations for Helium, Neon and Argon?
Noble Gases have complete shells and it makes it more convenient
He (2e-) = 1s²
Ne (10e-) = 1s² 2s² 2p^6
Ar (18e) = 1s² 2s² 2p^6 3s² 3p^6
2
New cards
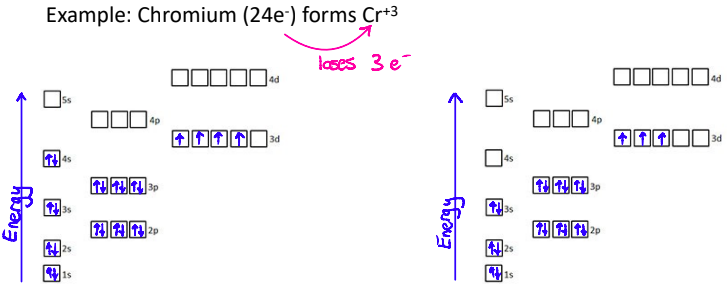
What is important about the 4s sub level?
Electrons are lost from the 4s sublevel first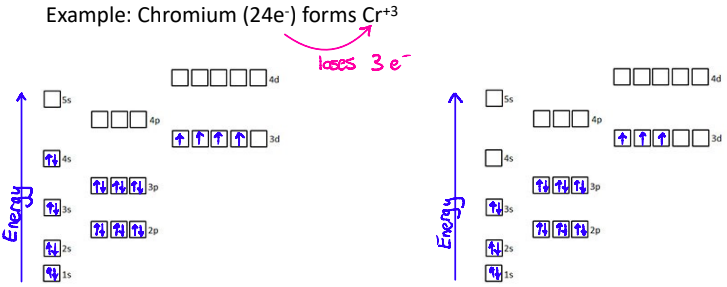
3
New cards
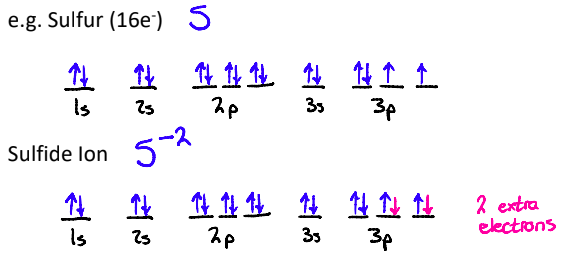
What do Electron Configs look like with Negative Ions?
4
New cards
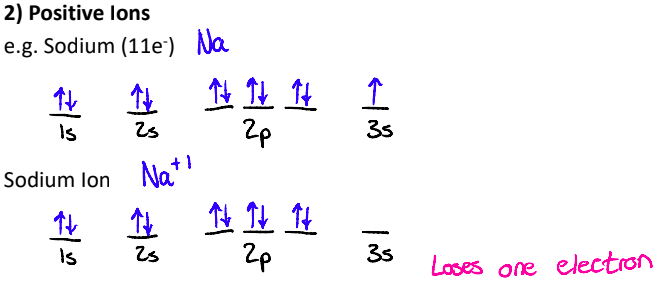
What do Electron Configs look like with Positive Ions?
5
New cards
What do Electron Configs look like with Transition Metals?
Transition metal ions don’t always follow the pattern of taking on the electron configuration of the closest noble gas
Transition metal electron configurations must be determined from the charge on the ion