biogeochem: nitrogen cycle
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
ammonia
NH3
ammonium
NH4+
Dinitrogen gas (and characteristics)
N2
relatively inert and inactive, most stable form, 78% of the atmosphere (largest reservoir)
Nitrous oxide
N2O
Nitric oxide
NO
Nitrite
NO2-
Nitric acid
HNO3
Nitrate ion
NO3-
describe the simple N cycle
N2 in atmosphere is fixed by bacteria where it’s used in organic compounds with amino groups (-NH2) in plants or animals. or decomposition of proteins into ammonia or converted into nitrate. the ammonia and nitrate go to plans. ammonia also goes through nitrification by bacteria and archaea to become nitrite which is nitrified into nitrate and nitrate goes through denitrification by bacteria into N2 in the atmosphere
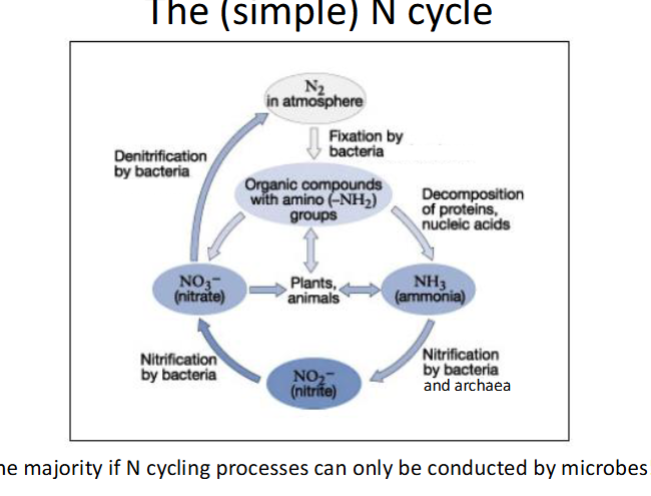
what are the 5 steps of the N cycle
nitrogen fixation
nitrification
assimilation
ammonification (also called mineralization or remineralization)
denitrification
what is nitrification
going from ammonium to nitrate via intermediate nitrite
what is denitrification
going from nitrates to atmospheric nitrogen
what is N2 fixation
making atmospheric nitrogen into a usable form (ammonia)
its performed by many prokaryotes.
biological N fixation is done by nitrogenase enzyme which is very oxygen sensitive.
cyanobacteria in aquatic systems and symbiotic N fixers in terrestrial
it’s very expensive and uses 16 ATP per N2 reduced
N fixing in oxic conditions
need to make little anoxic houses. multiple ways to protect nitrogenase:
moving to lower O2, making proteins to protect nitrogenase to oxygen exposure, form special cells to perform N fixation (nodules in plants, heterocytes in cyanobacteria) separation by time, fix at night when oxygen is lower due to decreased photosynthesis
types of N fixers
symbionts
rhizobia and legumes
actinomycetes and woody plants
associative
azotobacter
live in rhizosphere
free-living
cyanobacteria
also in soils
N assimilation and ammonification (aka mineralization)
assimilatory and dissimilatory
assimilatory:
uptake and transformation for biomass incorporations
biosynthesis, often energy requiring
dissimilatory
uptake and transformation for energy generation and storage
energy producing reaction
assimilation
formation of organic nitrogen compounds like amino acids from inorganic compounds. process where living organisms convert.
ammonium assimilation (“immobilization”
NH4+ update from decompositions and NH4+ generation from N fixation
preferred method for getting N for biosynthesis
easily kind to use - looks like kind to use in biomass
NO3- Assimilation
reducing NO3- to NH4 before incorporating
requires energy - better to just use NH4+
in a system with a lot of it - plants have adapted to just use it
mineralization (ammonification)
conversion of organic N (sugars, proteins) to inorganic forms
done as larger decomposition process = heterotrophs
mineralization is closely linked to decomposition
Nitrification
NH4+ to NO3-
ammonium to nitrate
two step process:
ammonia oxidation
nitrite oxidation
sets of organisms to do this are found together
who does ammonia oxidation? what are you converting?
NH4+ + O2 → NO2-
done by bacteria or archaea who are chemolithoautotrophs (means that this is how they make their living)
Nitrite oxidation
NO2- + H2O → NO3-
nitrite oxidase
nitrite to nitrate
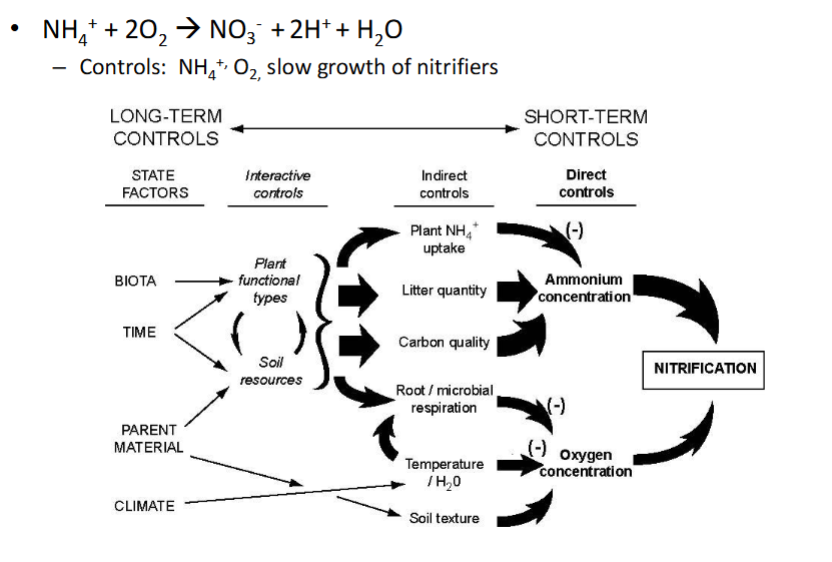
controls on nitrification
long term controls:
state factors: biota, time, parent material, climate
short-term controls:
direct controls: oxygen concentration and oxygen concentration
indirect controls:
plant NH4 uptake, litter quantity, carbon quality, root/microbial respiration, termperature, soil texture
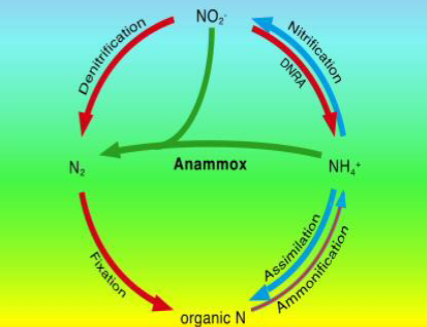
NO3- reduction processes
denitrification and annamox
denitrification
converts nitrate and nitrite into nitrogen gases and happens under anaerobic conditions
thought to be the predominant form of NO3- removal
cycles the reactive N back to N2 and removes limiting nutrient from environment
multi-step process where each step is conducted by a different enzyme
contributes gaseous byproducts include N2O which is a greenhouse gas
NO3- → NO2- → NO → N2O → N2
where there is higher oxygen levels or lower PH, there is poor activity of the nitrous oxide reductase so increases N2O production.
controls on denitrification
short term controls:
direct: nitrate concentration, labile carbon, oxygen concentration
indirect: plant NO3- uptake, litter quantity, carbon quality, room/microbial respiration, temperature and water, soil texture
long term:
state factors: biota, time, parent material, climate
Annamox (Anaerobic Oxidation of Ammonium)
Nitrite is combined with ammonium to remove combined N as N2
combines nitrification and denitrification
some organisms can do this and they are chemolithoautotrophic
strictly anaerobic process
discovered in wastewater from a dutch yeast factory
N reservoirs
atmosphere is the largest, not very available
rocks and sediments, not available
soils and vegetation, relatively available
N fluxes
N-fixation
denitrification
runoff/leaching
internal transformations and cycling are greater than fluxes between reservoirs
what are the biological and abiotic fluxes of the N cycle
biological: fixing, denitrification, nitrification
abiotic: industrial fixation, lightning fixation, fossil fuel and biomass burning, deposition
Reactive N in the atmosphere
NOx
NO, NO2
produced by combustion, denitrification, nitrification,
can remove zone
Nitrous oxide (N2O)
2nd most abundant N species
relatively unreactive
produced by denitrifiaction/nitrification
describe human alternation of N cycling
doubled the amount of reactive N on earth
three activities:
fertilizer productive
increase in N fixing crops
fossil fuel combustion
what is the haber-bosch process
discovered this to make bombs. makes fertilizer.
combines N with H at high temps
groundwater N contamination
toxic at high levels. possible carcinogen. causes blue baby syndrome
hyperthyroidism and diabetes