Topic 1: Inorganic Chemistry I - Ligands and Complexes
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Transition Metal Complexes
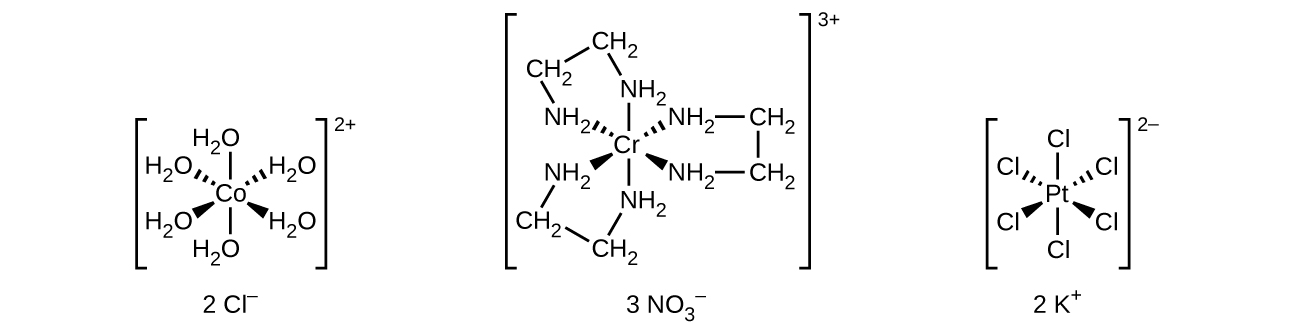
Transition metal ions are surrounded (coordinate bond) by molecules called ligands to form coordination compounds called complexes.
Formal Definition of a Complex
A compound is formally a complex when the coordination number is larger than the oxidation state of the center TM ion.
Example: [Cu(NH3)4(H2O)2]2+.
Complex Notation
Contain a complex within square brackets
Metal written first.
Ligands listed alphabetically.
Donor atom written first (e.g: water can be written as OH2).
Overall charge outside brackets.
In salts, cations are written before anions.
Counterions
Ions outside the square brackets are counterions. Transition metal complexes can be positively charged or negatively charged (or neutral) and the counterions balance that
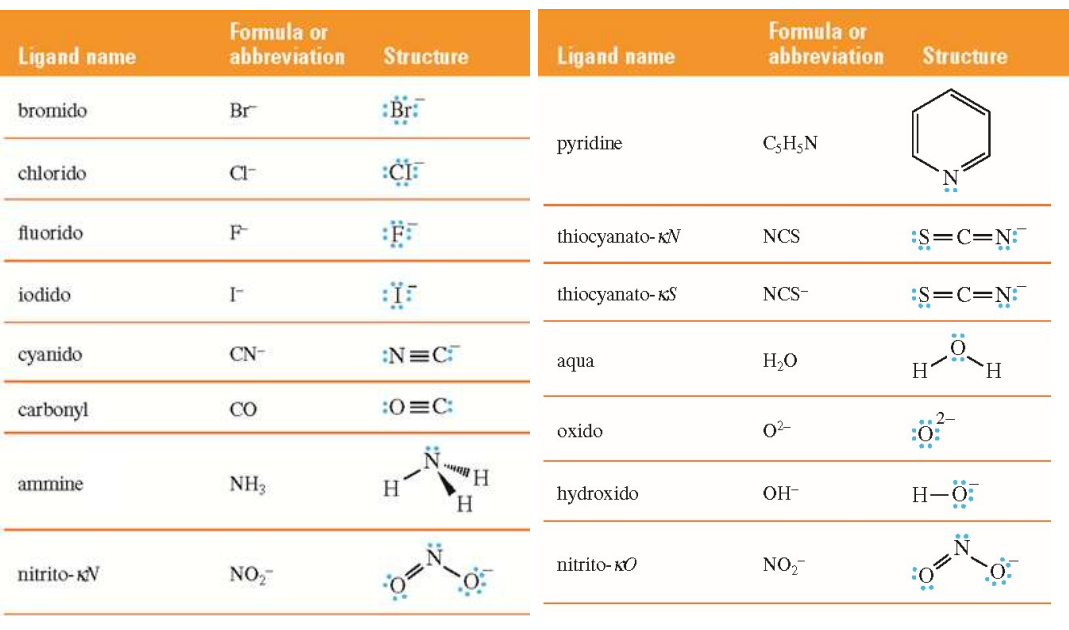
Ligand
A ligand is an ion or molecule that binds to a metal ion by donating a lone pair of electrons to form a coordinate bond.
Ligands as Lewis Bases
Ligands act as Lewis bases (electron donors), and the metal center acts as a Lewis acid (electron acceptor).
Donor Atom
The atom providing the lone pair to the metal is the donor atom.
Charges of Ligands
Ligands are usually neutral (e.g., H2O, NH3) or negatively charged (e.g., Cl−).
Denticity
Denticity is the number of coordinate bonds a ligand forms with a single metal center
Monodentate Ligands
Form one coordinate bond and occupy one coordination site
Multidentate Ligands
Form more than one coordinate bond with the same metal center (bidentate, tridentate, tetradentate, etc.).
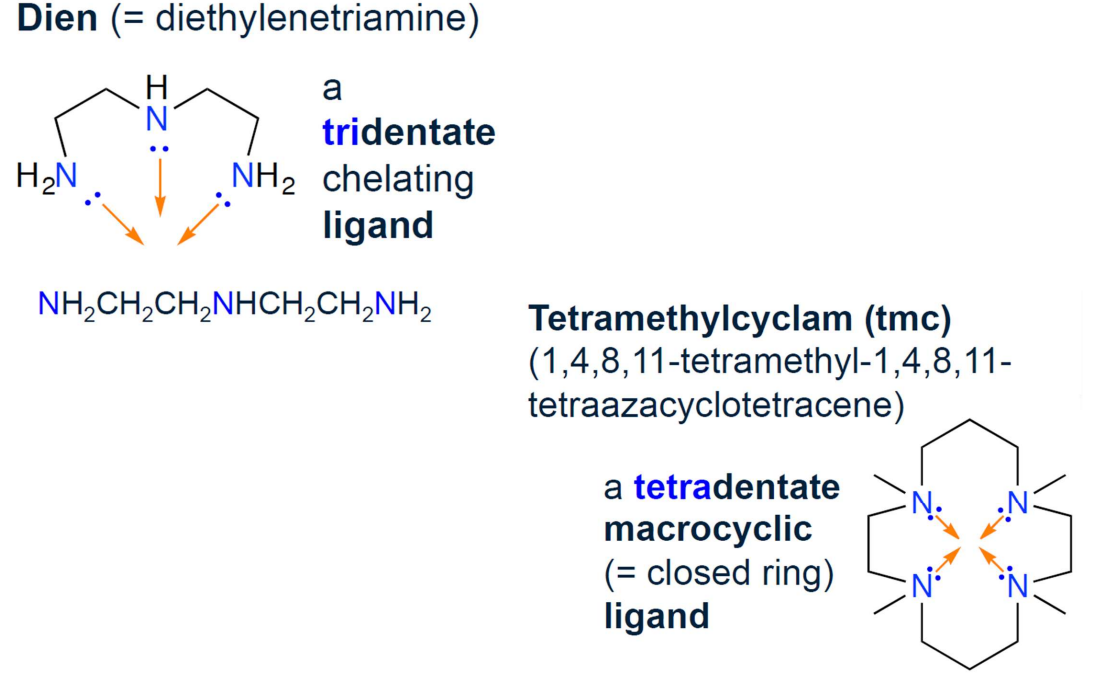
Chelate Complexes
Complexes formed by multidentate ligands are called chelate complexes.
Bidentate Ligands
Form two coordinate bonds with the same metal center
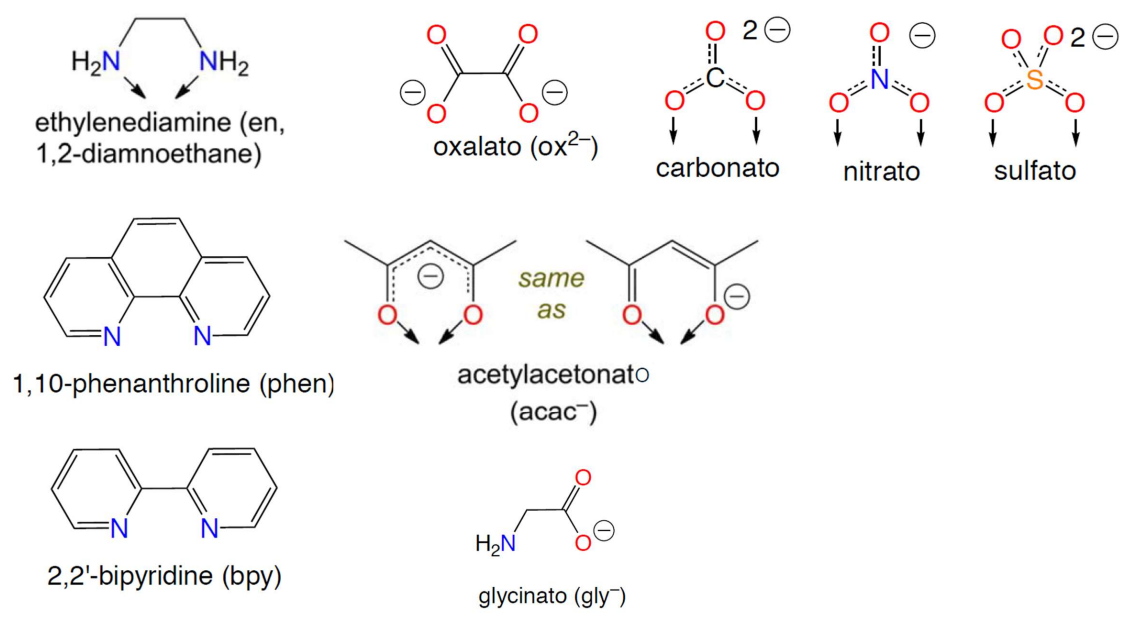
EDTA Ligand
Ethylenediaminetetraacetic acid (EDTA4−) is a hexadentate, forming six coordinate bonds (two nitrogens and four oxygens).
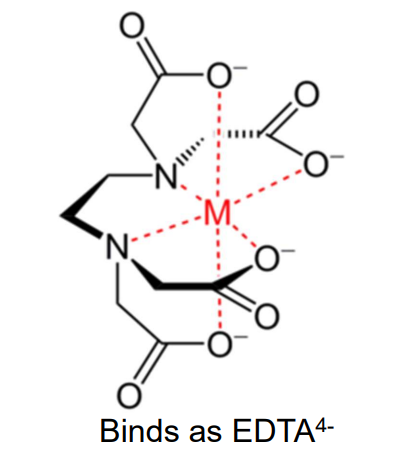
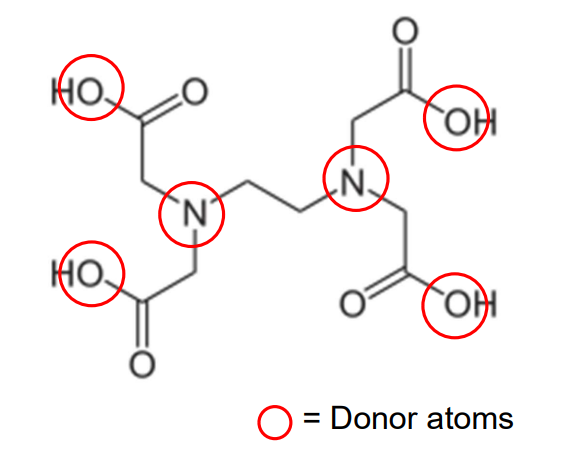
EDTA is used in chelation therapy to bind toxic metals for excretion from the body.
Ambidentate Ligands
Ligands that can bind through more than one atom
Example: Thiocyanate (SCN-) can bind through N or S, and nitrite (NO2-) can bind through N or O.
Kappa Notation
The Greek letter kappa (κ) denotes which donor atom is bound
Example: thiocyanato-κN means bound through nitrogen.
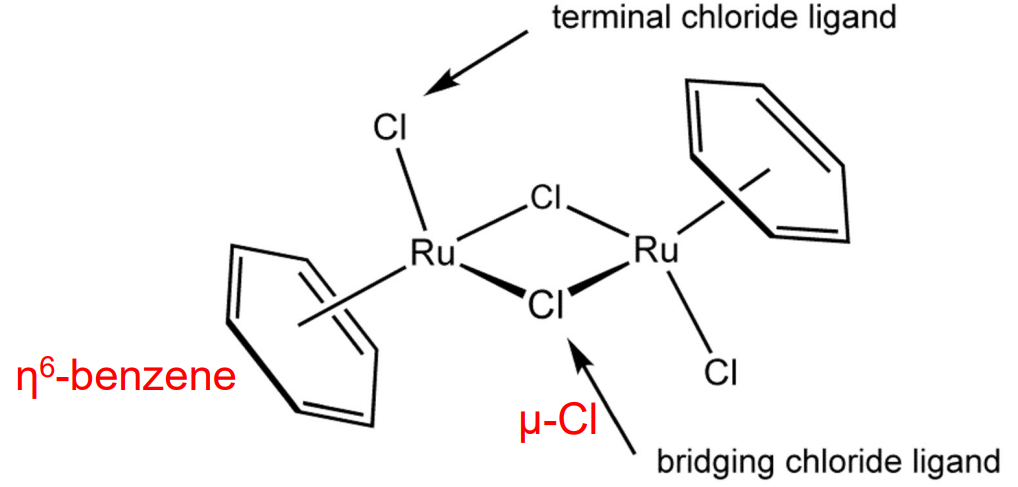
Bridging Ligands
Ligands that link two or more metal centers.
Denoted by Greek letter mu (μ).
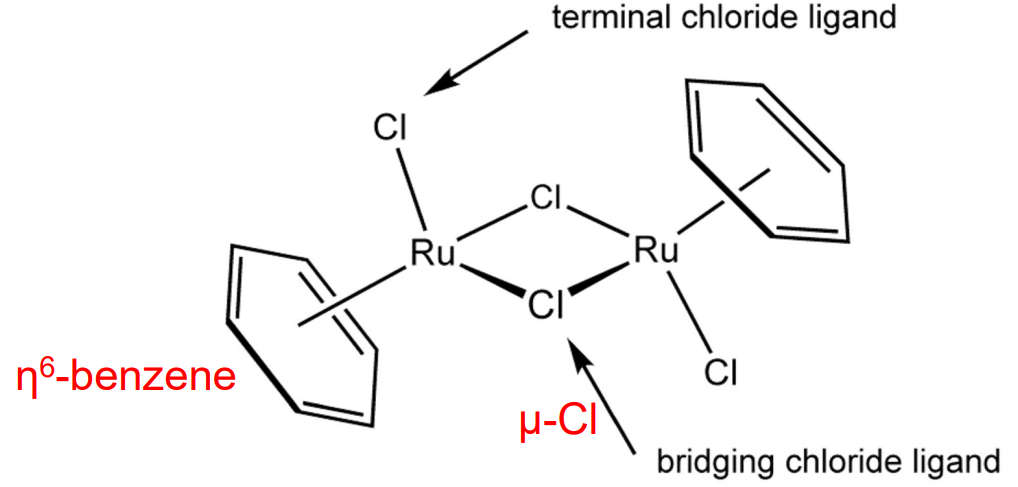
Hapticity Ligands
Eta (η) notation indicates how many contiguous atoms in a ligand are bound to the metal
Example: Benzene rings binds as η6 when all six carbons interact equally.
Coordination Number
Coordination number (CN) is the number of donor atoms directly bonded to the metal ion.
Geometry: CN = 2
Linear (180 degrees)
Rare
Found in d10 ions like Cu+, Ag+, Au+, Hg2+.

Geometry: CN = 3
Trigonal planar
Also rare
Found in Cu+ and Hg2+ (if not linear)
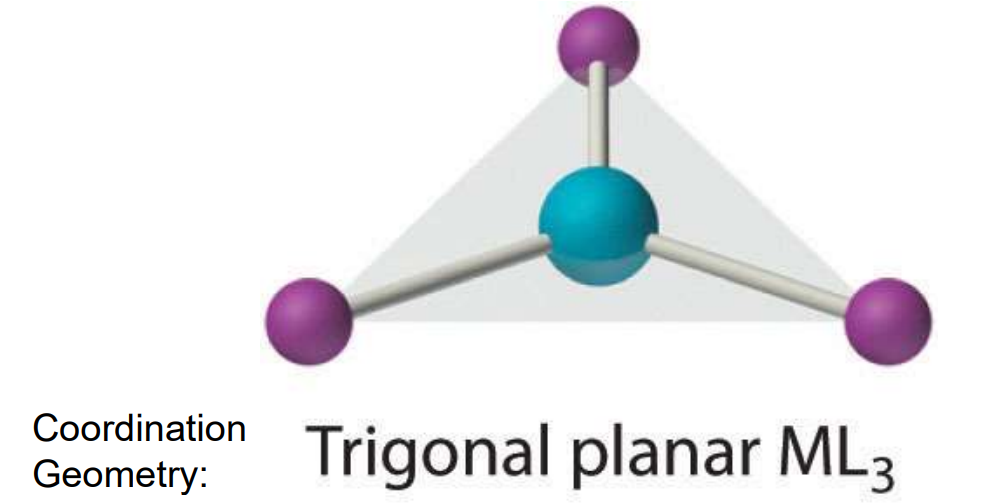
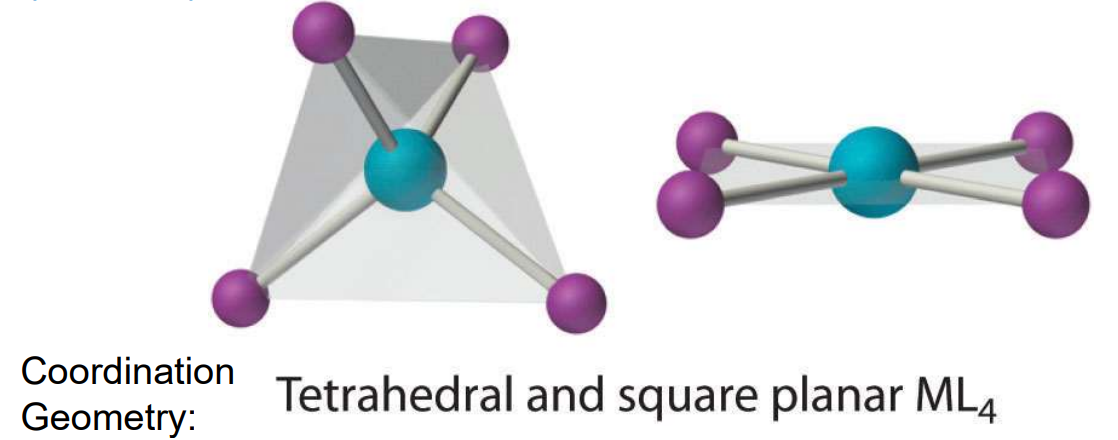
Geometry: CN = 4
Either tetrahedral or square planar.
Tetrahedral forms with halides ligands or d10 TM ions
Square planar common for d8 TM ions (Rh(I), Ir(I), Pd(II), Pt(II), Au(III), Ni(II)).
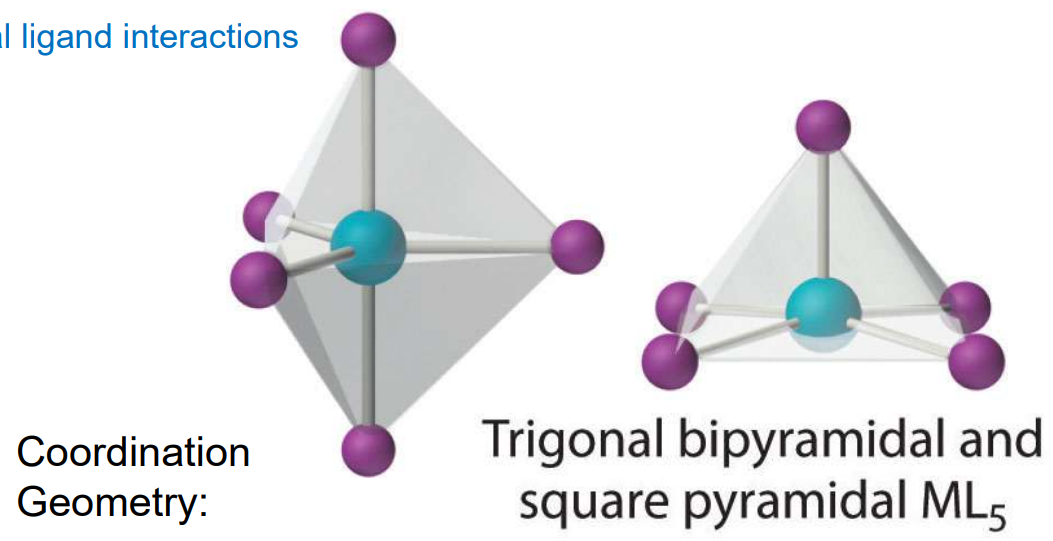
Geometry: CN = 5
Square pyramidal or trigonal bipyramidal
Rare, and in reality are often distorted shapes from metal-ligand interactions
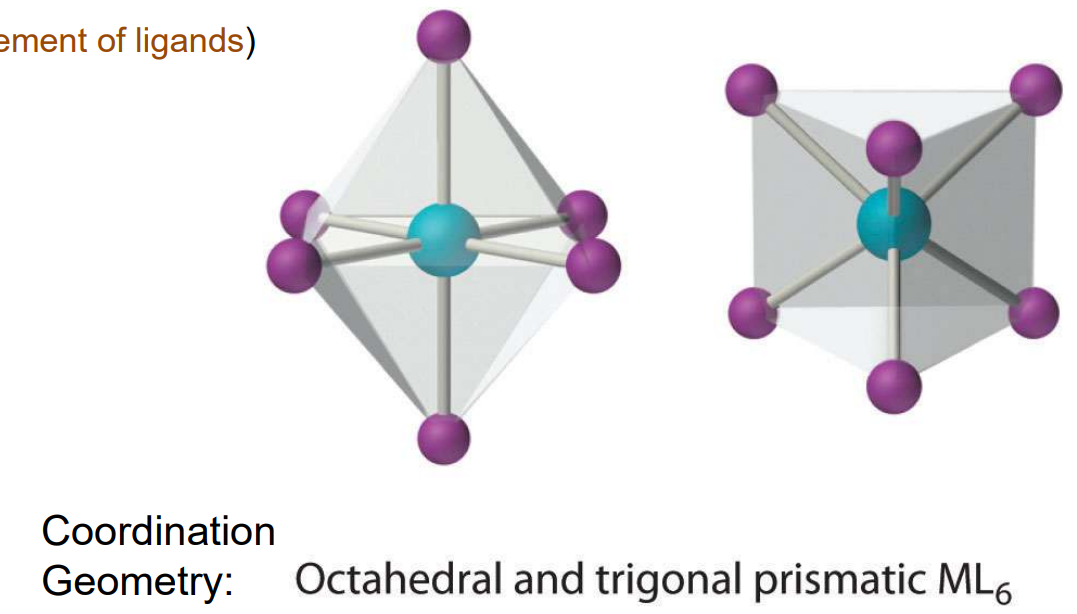
Geometry: CN = 6
Octahedral (most common) or trigonal prismatic (extremely rare – need ‘special’ arrangement of ligands)
IUPAC Naming Rules
Cation is named before the anion in an ionic coordination compound.
Within a complex, ligands are named first (alphabetically, ignoring prefixes), followed by the metal.
Metal oxidation state is shown in parentheses in Roman numerals.
Anionic ligands change their suffix: ide → ido, ite → ito, ate → ato.
Neutral ligands usually retain their names except: H2O = aqua, NH3 = ammine, CO = carbonyl, NO = nitrosyl.
Use prefixes: di-, tri-, tetra-, penta-, hexa- for simple ligands
Use prefixes: bis-, tris-, tetrakis-, pentakis-, hexakis- when ligand names already include a prefix.
Latin stems are used where available (e.g., iron → ferrate, copper → cuprate).
Charge of a Complex
In a neutral complex (neutral salt): (Amount of + charges) = (amount of - charges)
In a charged one: Charge of the metal (TM’s oxidation state) + ∑(charge on ligands)