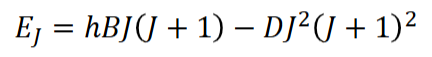rotational/microwave spectroscopy
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
in which regions are transitions between energy levels measured
microwave and far infrared
equation and units for wavenumber + why this is the case
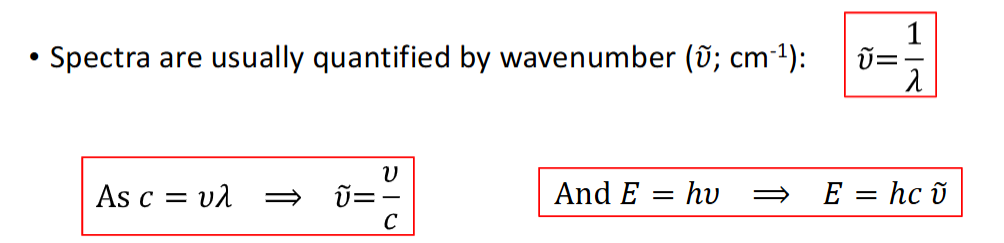
phase used for rotational spectroscopy and why?
quantities needed? how can this be achieved for the other phases?
usually done in the gas phase
solids inhibit rotation and too many collisions cause rapid energy transfer in liquids
sensitive technique so only small quantities of vapour are needed. many solids/liquids can be heated to give sufficient vapour pressure to record a spectrum
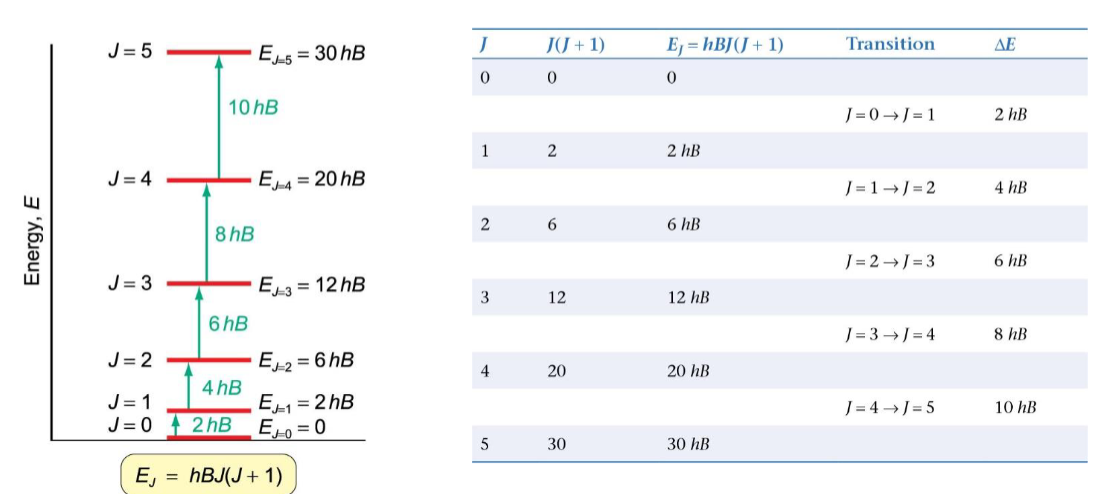
what does a rotational spectrum look like
energy?
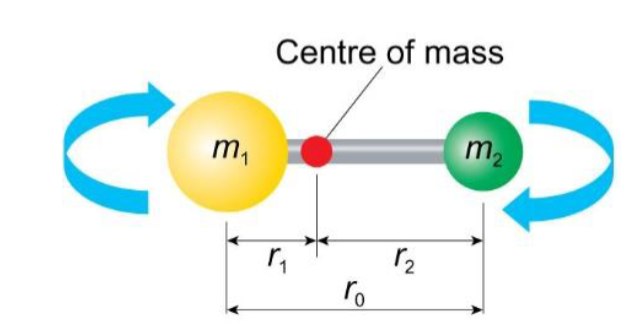
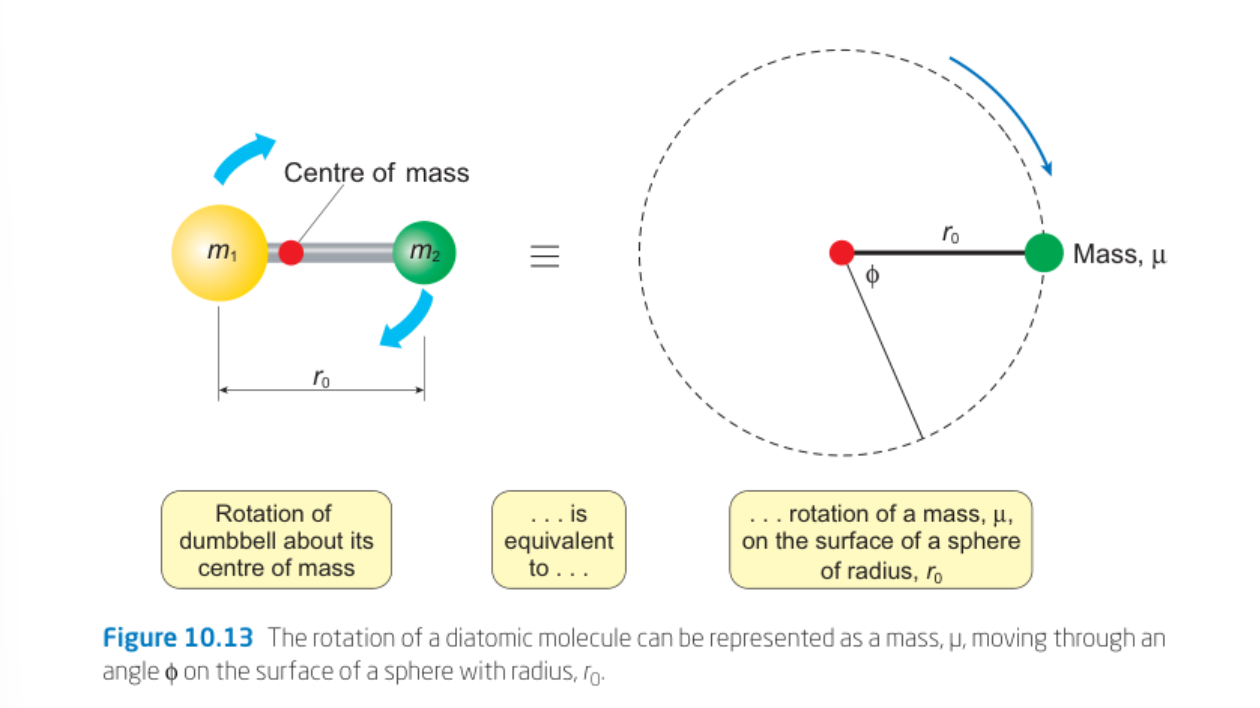
rigid rotor model
type of molecule?
assumption?
diagram
diatomic molecules
model assumes the bond length is fixed between the two atoms in a diatomic molecule, ie it does not change when the molecule rotates about its centre of mass.
moment of interia
symbol
equation
what it tells us
what does a higher value mean
symbol I
tells us how easy it is to spin the diatomic molecule about its centre of mass
larger masses or longer bond lengths mean the rotor will spin more slowly for a given magnitude of push. this means the moment of inertia is larger.
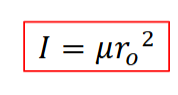
reduced mass symbol + equation
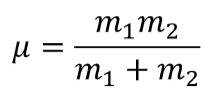
how can the rotation of a diatomic molecule be represented
quantisation of rotational energies
symbol
equation (not in terms of B) + what the symbols are
symbol EJ
J is the rotational quantum number and takes integer values J ≥ 0. EJ is then the energy of rotational level J
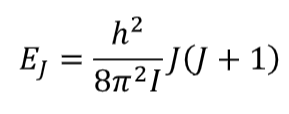
ground state for rotational energies
for J = 0, EJ = 0, so there is no non-zero ground state energy as there is for translational motion
rotational constant
symbol
equation
rewrite equation for EJ as a result
what does it depend on
symbol B
EJ = hBJ(J+1)
B is independent of J but depends on the chemistry of the molecule - each molecule has a unique value of B due to its moment of inertia I.
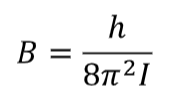
name the selection rules for rotational spectroscopy
gross selection rule
specific selection rule
gross selection rule
what is the case for diatomics
for a molecule to have a rotational spectrum it must have a permanent electric dipole. this is so that is has an oscillating electric dipole during rotation that can interact with the oscillating electric and magnetic fields of the electromagnetic radiation at the same frequency.
diatomics: homonuclear molecules have no permanent electric dipole and therefore no rotational spectrum eg Cl2, H2. heteronuclear diatomics do have a permanent electric dipole and do have a rotational spectrum, eg CO, NO.
specific selection rule
transitions can only take place between adjacent energy levels
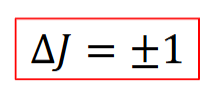

for J=0 to J=5 including energy level diagram
simplified rotation spectrum (don’t need to show intensity) showing ΔE and gaps between different ΔE values
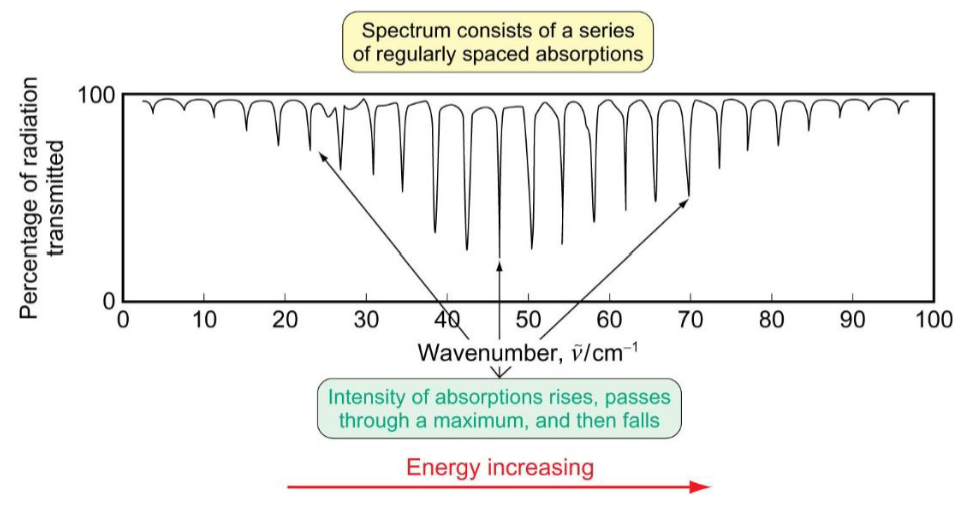
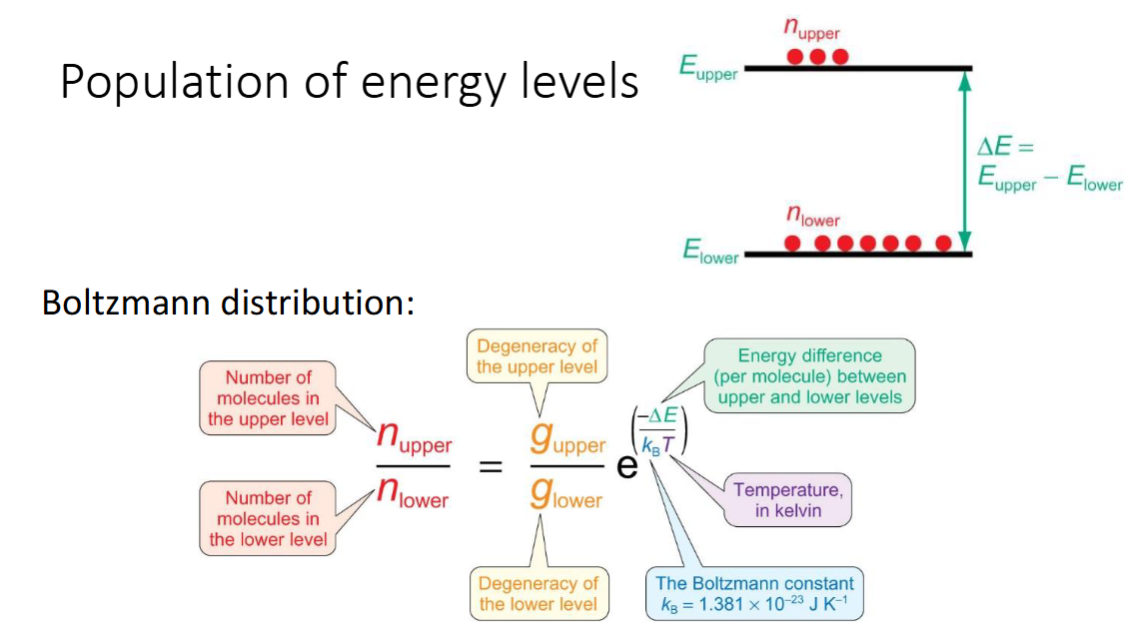
population of energy levels
name of distribution
equation and explain terms
diagram to show
degeneracy of rotational energy levels
(2J+1) distinct states for the Jth energy level
eg J = 0 has a spherical symmetric wavefunction similar to an s orbital
J = 1 has three wavefunctions similar to p orbitals of the H atom
J = 2 has five wavefunctions similar to the d orbitals of the H atom
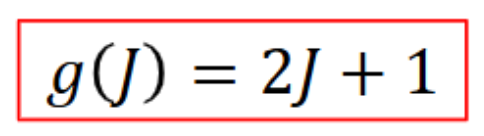
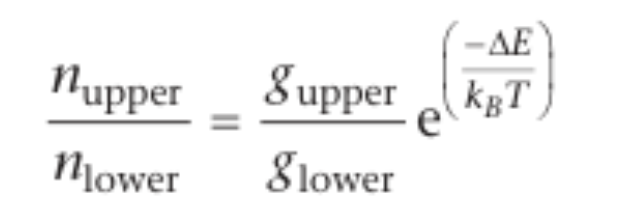
effect of degeneracies
ΔE is much less than kBT so the population for these levels is determined largely by the ratio of the degeneracies. although the degeneracy of the level increases as J increases, the value of ΔE also increases and eventually becomes larger than kBT - this is seen in the spectrum where the intensity increases to a maximum and then when ΔE ≥ kBT the exponential term decreases and the peaks get lower in intensity again - the ratio of adjacent peaks is greater than 1 before the maxium and less than 1 after.
how can temperature be estimated
ΔE can be obtained from the wavenumbers of the spectral peaks.
peak intensities allow calculation of energy level occupancies.
assigning peaks to specific energy levels allows calculation of degeneracies.
temperature is then the only unknown and the equation can be used to calculate it.
how can bond lengths be measured
ΔE = 2hB and ΔE = hv
the separation between the spectral lines (in frequency units) is equal to 2B
B is found (find separation and convert to Hz) and used to calculate moment of inertia I
calculate reduced mass
use moment of inertia and reduced mass to find bond length r0
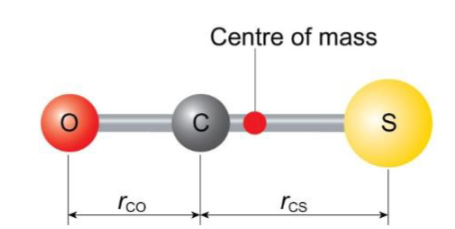
polyatomic molecules and simplest system
molecules with more than two atoms that have a permanent dipole give rise to rotational spectra similar in appearance to those of diatomics, however the interpretation of the data is much more complex
simplest linear example (after diatomics) is OCS
non-rigid rotors
why this model?
what happens as EJ increases
equation accounting for this?
assuming the bonds are rigid and of fixed length is not always valid
as rotational energy increases, the molecule spins faster and the length can increase - centrifugal distortion
this is accounted for by an extra term in the energy of rotation - D is the centrifugal distortion constant
as rotational energy increases, the gaps between the energy levels get smaller