PHAR303 - Toxic Responses of the Liver
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
Name some of the major functions of the liver that can be perturbed by exposure to toxicants.
1. Protein synthesis (e.g. clotting factors, resulting in excess bleeding).
2. Metabolic function (e.g. glucose, resulting in hypoglycemia).
3. Bile production (e.g. dietary lipid absorption, resulting in diarrhea and malnutrition).
4. Detoxification (e.g. xenobiotics, resulting in diminished detoxification).
The liver is an important line of defence against...
potentially harmful xenobiotics
It was estimated that around 33% of the 677 most-common workplace chemicals are associated with hepatotoxicity. True or false?
True.
Where does most of the blood from the liver come from/flow to? What are some characteristics of this blood?
Approx. 70% of the blood comes from the hepatic portal vein, which drains the stomach and the intestines.
This blood is oxygen poor, but nutrient-rich.
Which blood vessel supplies the liver with blood?
Approx. 30% of blood comes from the hepatic artery.
This blood is oxygen-rich.
What are the main characteristic of the hepatic portal system?
It is a portal system: it has two capillary beds connected by a vein (begins and ends in capillaries).
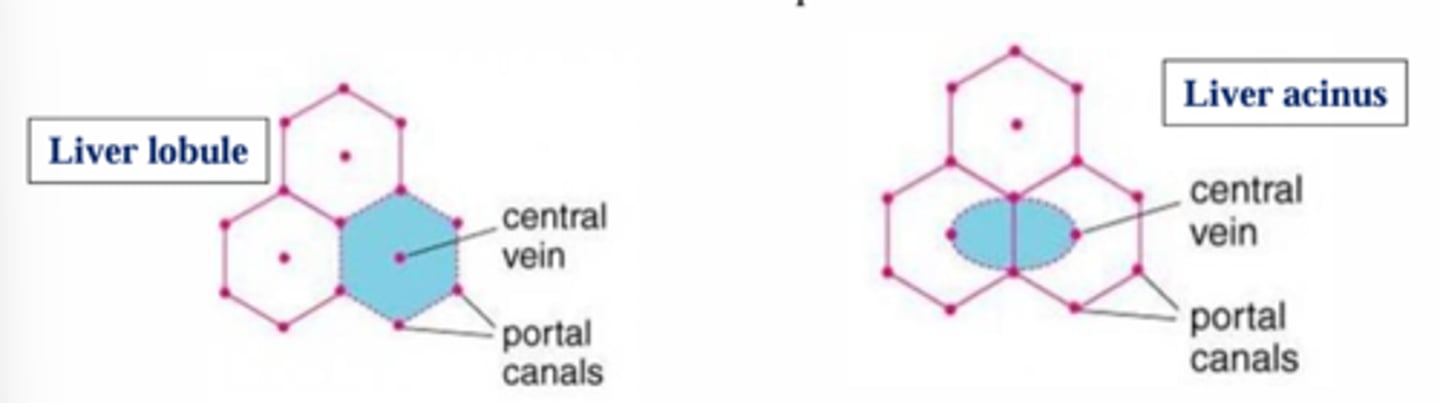
What is the basic unit of the liver?
The lobule.
What is the main structure of the liver lobule?
It is like spokes on a wheel: The hepatic (portal) triad.
A central vein is surrounded by hepatic portal triads.
There are hundreds of thousands of liver lobules in the typical adult liver. True or false?
True.
What makes up the hepatic portal triad?
hepatic portal veins
hepatic arteries
small branches of bile ducts.
Where does blood enter and exit the liver lobule?
It enters and exits via the sinusoids.
What are the types of cells in the liver?
Hepatocytes,
Endothelial cells,
Kupffer cells,
Ito cells.
What are the main characteristics of hepatocytes?
They make up 65% of the liver,
They are one cell layer thick that is separated by liver sinusoids
What are sinusoids?
channels between chords of hepatocytes where blood percolates (enters and exits), allowing for exchange of factors between endothelial cells and hepatocytes on the way to the hepatic vein.
What are the main characteristics of liver endothelial cells?
They line the sinusoids;
They make up 16% of the liver.
What are the main characteristics of Kupffer cells?
They make up 12% of the liver.
They are hepatic macrophages that engulf pathogens, cell debris, and damaged blood cells.
What are the main characteristics of Ito cells?
They make up 8% of the liver.
They are also known as hepatic stellate cells.
They store vitamin A.
What is the space of Disse?
The space between hepatocytes and sinusoids which contains stellate cells, blood plasma, lymph, and extracellular matrix (e.g., collagen)
What is the smallest functional unit of the liver?
The hepatic acinus.
What is the difference between the hepatic acinus and the hepatic lobule?
The difference between the acinus and the lobule is that the acinus is the smallest functional unit, whereas the lobule is a structural unit.
What are the characteristics of the hepatic acinus?
They are oriented around the vascular system, located between 2 central veins, and 2 portal triads.
The acinus is divided into 3 zones that correspond to the distance from blood supply.
What are the characteristics of zone 1 of the hepatic acinus?
Zone 1 (periportal):
closest to the arterioles,
best oxygenated,
higher glutathione,
ammonia detoxification,
fatty acid oxidation.
What is zone 2 of the hepatic acinus?
The intermediate/mid-lobular zone.
What are the characteristics of zone 3 of the hepatic acinus?
Zone 3 (perivenous/periventral):
Farthest from the arterioles,
least oxygenated,
xenobiotic metabolism: higher CYP450s, especially CYP2E1.
One can distinguish acinus zoning by using...
staining methods.
Synthesis of serum protein seems to be zone-specific.
False. Synthesis of serum protein does not seem to be zone-specific.
What are factors that influence liver toxicity?
The Zones can be differently affected by toxicants
> Zone 3 has more P450s compared to other zones. This zone is where the xenobioic is first activated into something noxious
Uptakes and concentrations
> fenestration in the sinusoids enables close contact with sinusoids causing lipophilic concentration
> Toxins may be substrates for sinusoidal transporters
Activation of the sinusoidal cells, such as Kupffer cells
> inflammatory and immune responses can exacerbate the injury
Liver injury due to toxicants can depend on which factors?
Intensity of the insult,
Population of cells affected,
Chronic vs acute exposure.
Which toxic effects cause hepatocellular dysfunction and damage?
Toxicants that block uptake, secretion, or bioactivation.
Disruption of which pathways kills hepatocytes due to acute damage (ex. increased ROS)?
Membrane integrity,
Mitochondrial functions,
Cytoskeleton,
Transporter and enzymes.
What is the most common cause of liver injury due to chronic damage (ex. repeated insults)?
Scar tissue in the damaged areas.
Hepatocyte cell death is a consequence of...
Toxicant expsoure
What are the two mechanisms that cause hepatocyte cell death?
Apoptosis and necrosis
What is the most common type of zone necrosis?
Zone 3 necrosis.
What is bile?
Bile is a yellowish fluid that contains: Bile acids, GSH, phospholipids, cholesterol, bilirubin and xenobiotics
Bile is the primary pathway for:
1. Elimination of bilirubin; product of the catabolic process that breaks down heme
2. Excess cholesterol
3. Xenobiotics that are not water soluble enough for excretion in the urine
Bile is needed for:
Digestions and absorption of lipids from the gut
Bile formation requires:
Well-functioning hepatocytes
An intact biliary tree
What is cholestasis?
A reduced or stopped bile flow.
It may be due to a decrease in the volume of bile formed or an impaired secretion of specific solutes into bile.
What two types of pathologies does cholestasis include?
Hepatocellular cholestasis
Canalicular cholestasis
What is hepatocellular cholestasis?
Bile accumulation in the cytoplasm of liver cells.
What is canalicular cholestasis?
Bile accumulation in the canaliculi.
How is cholestasis characterized?
Characterized by the increased serum levels of bile components (e.g. bile salts and bilirubin)
What some clinical symptoms of cholestasis?
Jaundice, where bilirubin accumulates in the skin and eyes.
It also spills into the urine, resulting in a bright yellow to dark brown urine.
What are the two types of cholestasis and what are they caused by?
Extrahepatic/obstructive: bile flow is impeded by lesions (e.g. gallstones, neoplasm)
Intrahepatic: Cause relates to damage to hepatocytes and/or biliary canliculi (e.g. viral hepatisitis, drugs, chemicals)
What is the general mechanism of hepatobiliary transport?
A small amount of bile acid is produced every day by CYP7A1.
Most bile is recycled by enterohepatic circulation.
A small amount of bile is broken down and excreted via canalicular excretion.
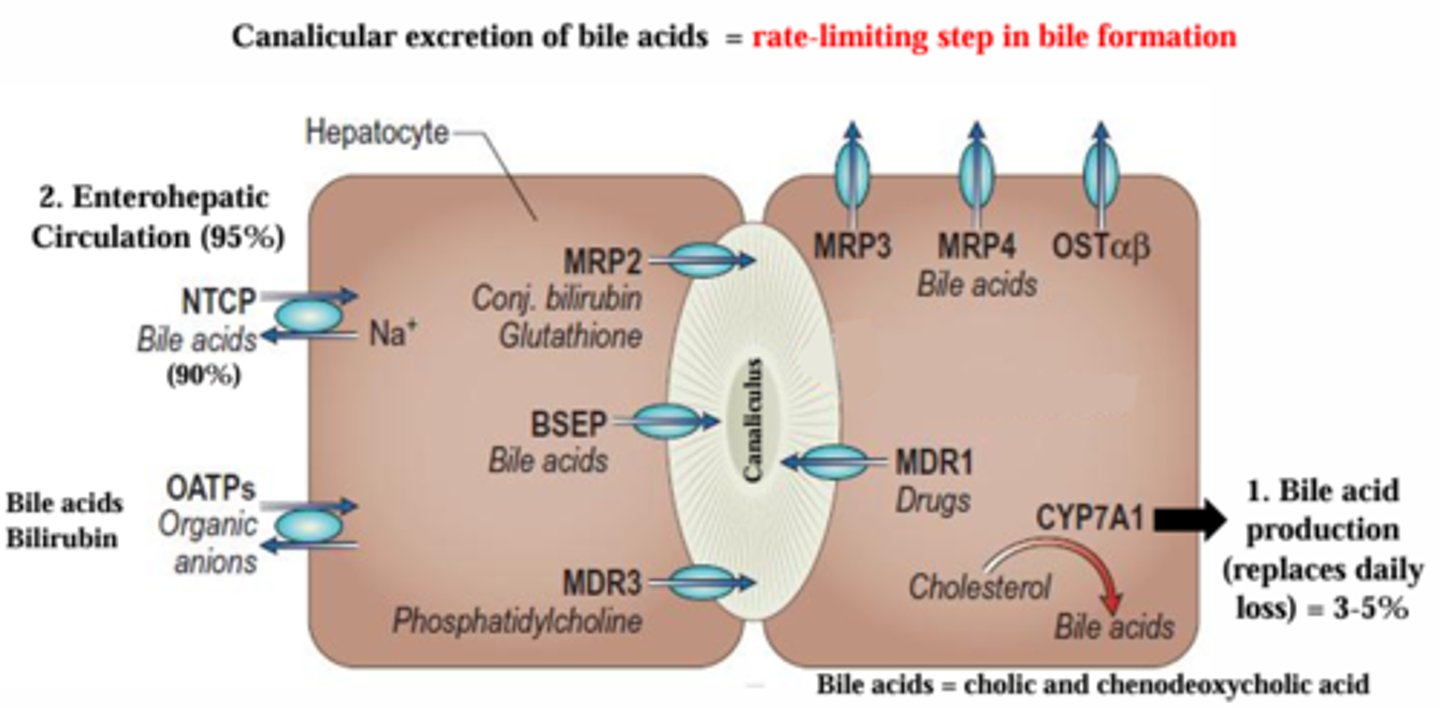
How do hepatocytes take up bile acids and other compounds to form bile?
Two main transporters:
Sodium taurocholrate co-transporter (NTCP) = uptake of bile acids
Organic anion transporter (OATP) = uptake of bilirubin
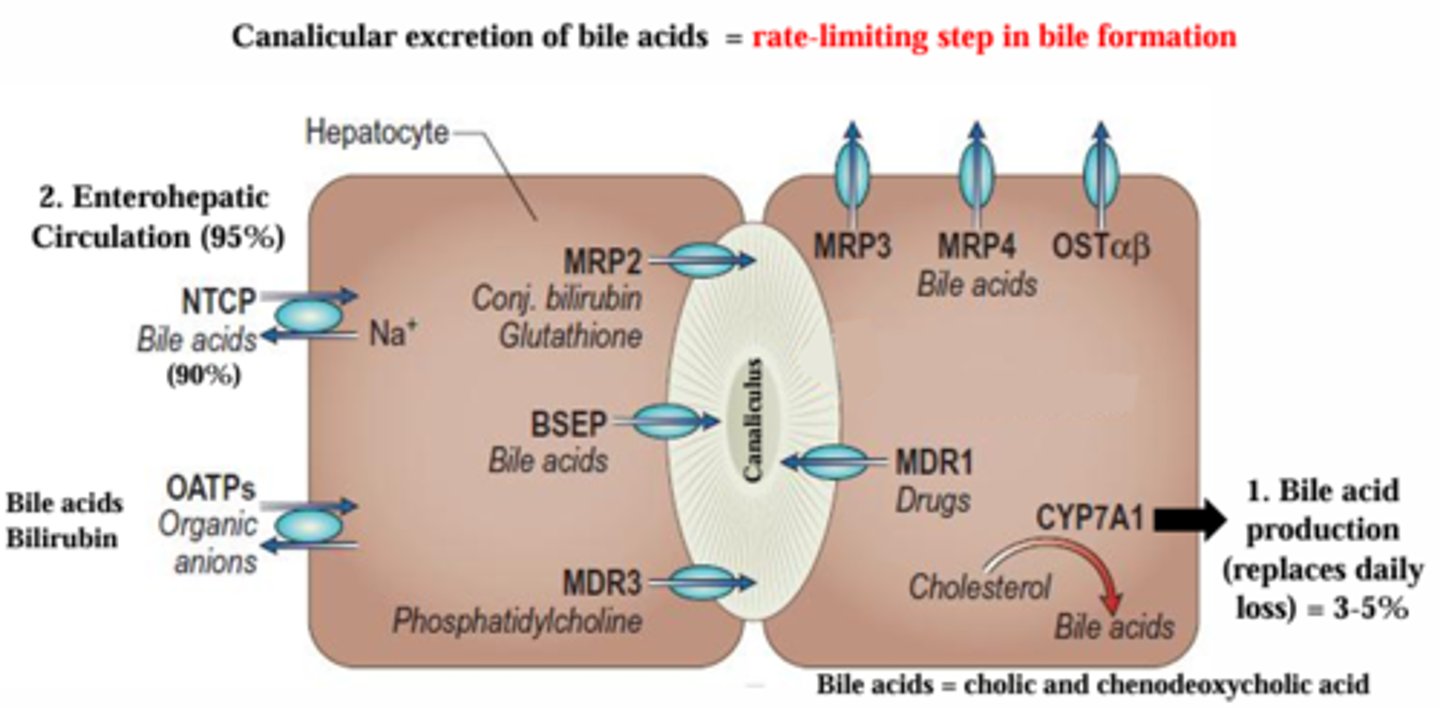
What is the rate-limiting step in bile formation?
Canalicular excretion of bile acids via the BSEP (bile salt export pump), MRP2 and MDR3 (which are multidrug resistance related/associated protein). These are bile salt export transporters located on the canalicular side.
What are some potential mechanisms for cholestasis?
Impaired uptake,
Diminished contractility of the canaliculus,
Leaky paracellular junctions,
Impaired secretion,
Concentration of reactive species.
Diminished transcytosis,
>Transcytosis: extracellular cargo is endocytosed, shuttled across the cytoplasm in membrane‐bound vesicles, and secreted at a different plasma membrane surface.
Which medication is known to cause cholestasis? By what mechanism?
Chlorpromazine may cause cholestasis by diminished contractility of the canaliculus, and impaired uptake via regulation of NTCP and BSEP in 1-2% of patients.
What is cholangiodestructive cholestasis? Which structures are affected?
Damaged to the bile ducts that carry bile from the liver to the GIT.
Damage is to either the intrahepatic bile ducts or the biliary epithelium. Causes chronic cholestasis.
True or false: lesions of the biliary tree can result in...
1. bile duct obstruction
2. lesions can be acute or chronic
3. vanishing bile dut syndrome: loss of bile ducts if damage is extensive enough
Cholangiodustructive cholestasis can be caused by infectious disease or drug induced. True or false.
True.
What is paraquat?
Widely used herbicide.
What is the most common exposure of paraquat?
Ingestion (delibarate or accidental)
Which diseases are caused by paraquat?
Acute toxicity: lung disease.
Chronic toxicity: prolonged cholestasis after paraquat poisoning.
What is the proposed mechanisms of paraquat toxicity?
ROS production, leading to cell death and organ injury. May also involve activation of other transcription factors like NF-kB.
What are the different stages of liver disease?
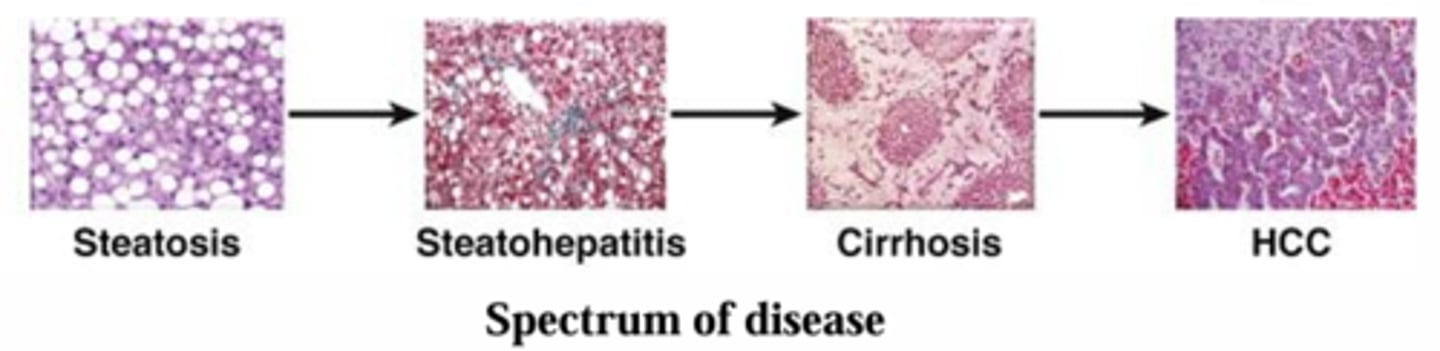
What is hepatic steatosis, or fatty liver?
The buildup of triglycerides in the hepatocyte, leading to >10% fat content in the liver.
What are the agents which may cause hepatic steatosis?
TASH (toxicant-associated steatohepatitis)
e.g. CCl4
ASH (alcoholic steatohepatitis)
NASH (non-alcoholic; often associated with a "Western" or high-fat diet steatohepatitis)
What is the general pathways for steatosis?
Influx of fatty acids from lipolysis of adipose tissue, and dietary fats.
De novo lipogenesis,
Decrease in triglyceride secretion,
Decrease in fatty acid oxidation.
What is CCL4?
CCl4 is a clear liquid with a sweet smell that evaporates very easily.
Manufactured – does not occur naturally in the environment.
Previously used in fire extinguishers, pesticides, insulating foams, dry cleaning solvent, Freon production (refrigerators).
Used to produce chlorofluorocarbon refrigerants.
Group 2B carcinogen (IARC)
What is the primary route of exposure for CCl4?
Inhalation
Besides the liver, what other organs does CCl4 affect?
Kidney (less urine, kidney failure)
Nervous system (high levels; signs of intoxication)
What step is required for CCl4 toxicity? What is the general mechanism of toxicity of CCl4?
Metabolic activation by CYP2E1 in hepatocytes is required.
CYP2E1 converts CCl4 to tricholromethyl radicals which cause GSH depletion and disrupted lipid homeostasis/secretion, resulting in steatosis.
CCl4 has a risk of synergy with...
alcohol as it induces the expression of CYP2E1
Excessive alcohol consumption causes...
alcoholic liver disease (ALD) which encompasses steatosis, steatohepatitis, cirrhosis, and HCC.
Alcoholic steatosis is associated with the following conditions within the cell:
1. decreased fatty acid oxidation
2. increased TAG synthesis and accumalation in Zone 3
True or false: alcoholic fatty liver is irreversible, even with cessation of consumption of alcohol.
False.
Steatosis is asymptomatic and reversible if alcohol consumption ceases.
What are the steps of progression to hepatocellular carcinoma? Are these steps reversible?
Steatosis (asymptomatic, reversible),
Fibrosis (can be reversible),
Cirrhosis (mostly irreversible),
HCC. (not reversible)
Steatohepatitis is an intermediate that accompanies fatty liver and is a prerequisite for progression to fibrosis and cirrhosis. Includes fatty liver, inflammatory cells and hepatocelluar damage.
What is the role of PPAR-alpha in steatosis?
PPARα prevents steatosis because it can transcriptionally increase several of the enzymes important to fatty acid metabolism.
PPARα expression is reduced by acetaldehyde.
Inhibition of fatty acid oxidation and export.
What is the role of SREBP1c in steatosis?
SREBP1c (sterol regulatory element-binding protein 1c) is important for the upregulation of a number of different genes and proteins which are essential to lipogenesis.
Increased by acetaldehyde and TNF-α.
What causes DAMPs and what is the role of DAMPs in steatosis?
Heightened oxidative stress and steatosis can cause cellular death, either by necrosis or apoptosis, and damage-associated molecular patterns (DAMPs) releases.
DAMPs can activate Kupffer cells, which will produce more ROS and inflammatory cytokines.
What is the mechanism of toxicity of alcohol in the hepatocyte?
Chronic alcohol induces CYP2E1, up to a 10-20-fold increase.
Initially, alcohol is metabolized by Alcohol dehydrogenase, followed by acetaldehyde dehydrogenase.
The increase of CYP2E1 causes an accumulation of acetaldehyde, which results in the depletion of GSH, increasing ROS. Increased ROS decreases PPAR-alpha, blocking fatty acid oxidation and export. It also increases SPREBP1c, increasing lipogenesis.
The fat accumulation causes steatosis, which causes cell death and necrosis. It also results in the activation of DAMPs, which promote inflammation and hepatocyte injury. Chronic alcohol use also leads to poor nutrition (see also Lecture 11) associated with the disruption of the integrity of intestine, leading to a release of bacterial components like lipopolysaccharide (LPS). LPS is able to activate Kupffer cells (immune cells in the liver) which can also lead to inflammation.
What is hepatic fibrosis?
It occurs when normal tissue is replaced by scar tissue.
Hepatic fibrosis is characterized by increased extracellular matrix that scars the liver. May be reversible
What is the the key event in hepatic fibrosis?
Hepatic stellate cell activation.
What is the most effective treatment for fibrosis?
removal of the causative agent.
What is the major source of hepatic fibrosis?
The ECM in the liver. HSC is the major source of ECM in the liver (they differentiate into a myofibroblast-like cell which can potently synthesize ECM).
Chronic damage from which sources cause hepatic fibrosis? What is the mechanism behind them?
Acetaldehyde/ROS,
Free radicals from CCl4,
Myofibroblasts and macrophage migration and release of TGF-beta, which triggers the differentiation of HSC to myofibroblasts. The myofibroblasts then secrete collagens and other ECM proteins leading to cirrhosis.
What is the main characteristic of alcoholic cirrhosis?
The diffuse process is characterized by fibrosis and conversion of normal liver architecture into structurally abnormal nodules.
True or false: cirrhosis is a pre-neoplasic lesion.
True.
What is the single greatest risk factor for hepatocellular carcinoma?
Cirrhosis.
Which polymorphisms are protective against liver cancer?
ADH1B2 and ALDH2/2, mutations in acetaldehyde dehydrogenase are protective against alcohol dependence, and thus liver cancer.
Alcohol consumption with these mutations result in flushing syndrome, or a disulfiram reaction.
What is hepatic angiosarcoma?
Originating from endothelial cells, it is a rare malignancy of the liver.
It is associated with vinyl chloride toxicity.
What is vinyl chloride?
VC is a colorless gas with a mild sweet odor used in commercial production of PVC plastics.
Does not bioaccumulate. IARC Group I carcinogen:
HCC (VC and alcohol synergize)
Hepatic angiosarcoma (hemangiosarcoma)
What is the main exposure route for vinyl chloride?
Exposure is primarily through inhalation; also dermal.
Rapidly absorbed through the lungs and metabolized in the liver.
What is the brunt of the injury from vinyl chloride toxicity?
The brunt of the injury is at the sinusoidal level.
What is the mechanism of toxicity of vinyl chloride?
Metabolic activation of VC by CYP2E1 results in the formation of chloroethylene oxide.
In hepatocytes, CEO forms adducts with DNA; it is repaired while CEO is detoxified by enzymes.
In endothelial cells, CEO forms adducts with DNA, causing mutations and resulting in angiosarcoma, since there are fewer repair mechanisms and a higher replication rate.
What is the mechanism of toxicity of acetaminophen?
At toxic doses, acetaminophen is converted by CYP2E1 into NAPQI, a toxic metabolite. It causes GSH depletion, which causes hepatic injury and hepatic cell death (zone 3 necrosis).
Which drug increases the toxicity of acetaminophen? Via which mechanisms?
Alcohol increases the toxicity of acetaminophen.
Alcohol increases CYP2E1 activity, increasing the conversion of acetaminophen to NAPQI.
Alcohol depletes GSH, sensitizing individuals to acetaminophen poisoning.