Chemistry Prefixes, Suffixes, and Hydrocarbons
1/27
Earn XP
Description and Tags
Vocabulary flashcards summarizing key chemistry prefixes, suffixes, and common hydrocarbons discussed in the lecture notes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
inter-
Prefix meaning between
intra-
Prefix meaning within
meth(yl)-
Prefix for an organic group containing a single carbon (CH3-)
hydro-
Prefix relating to water
mono-
Prefix meaning one
poly-
Prefix meaning many or multiple
-ide
Suffix indicating a binary compound of two elements; typically a non-metallic ion
-ane
Suffix denoting an alkane; hydrocarbon with only single bonds
-ene
Suffix denoting an alkene; hydrocarbon containing a carbon–carbon double bond
-yne
Suffix denoting an alkyne; hydrocarbon containing a carbon–carbon triple bond
-philic
Suffix meaning having an affinity for
-phobic
Suffix meaning having an aversion to; repelled by
-ose
Suffix indicating a sugar
-ase
Suffix indicating an enzyme
-ite
Suffix indicating an oxyanion with one fewer oxygen than the -ate form
-ic acid
Suffix denoting a carboxylic acid group
-ate
Suffix indicating a polyatomic ion composed of three or more elements, one of which is oxygen
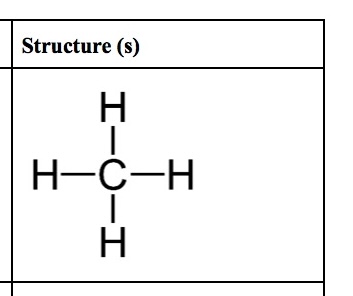
Methane
Simplest alkane; formula CH4
Ethane
Two-carbon alkane; formula C2H6
Propane
Three-carbon alkane; formula C3H8
Butane
Four-carbon alkane; formula C4H10
Pentane
Five-carbon alkane; formula C5H12
Hexane
Six-carbon alkane; formula C6H14
Heptane
Seven-carbon alkane; formula C7H16
Octane
Eight-carbon alkane; formula C8H18
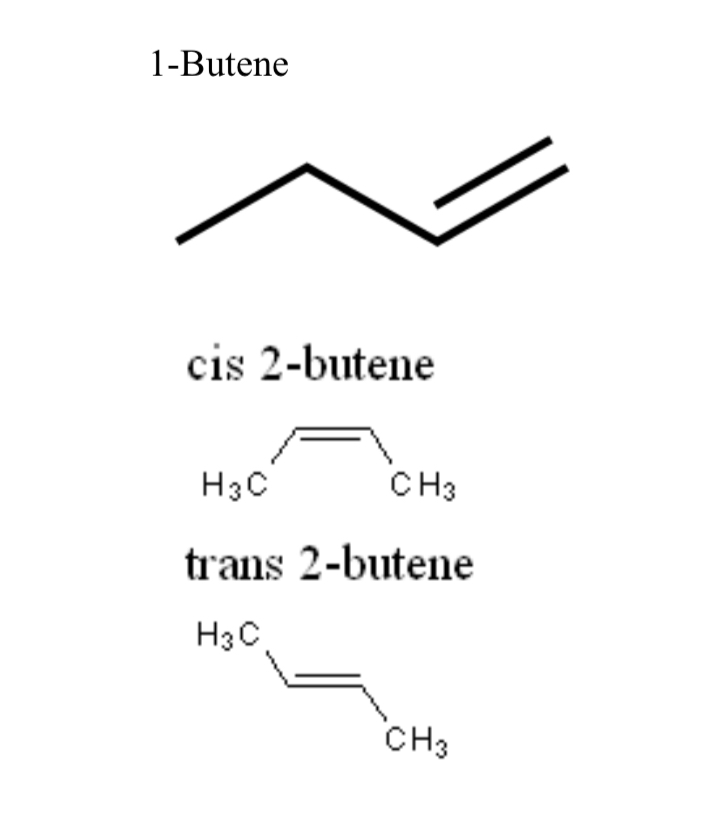
1-Butene
Four-carbon alkene with a double bond between carbons 1 and 2
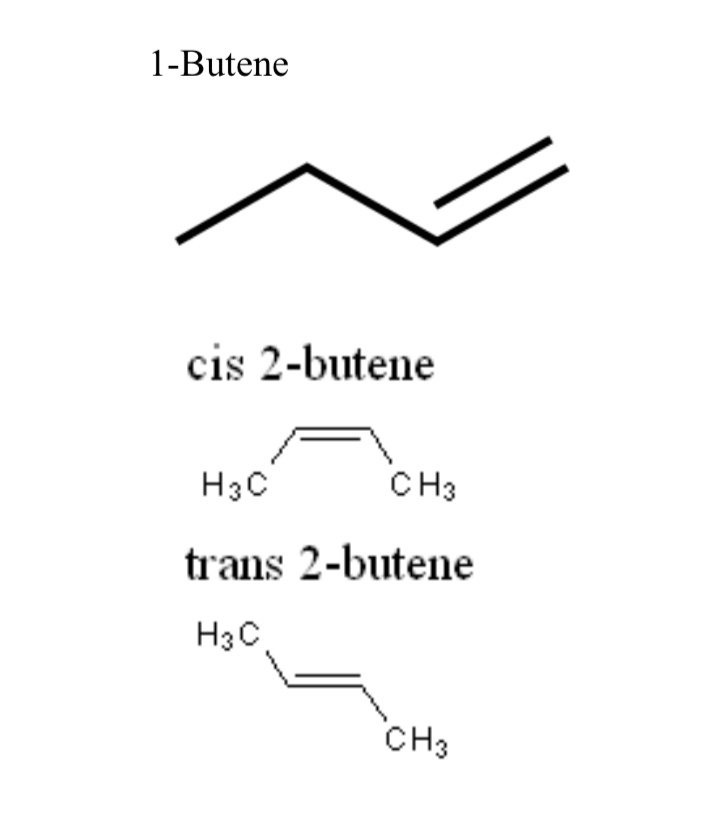
cis-2-butene
Geometric isomer of 2-butene with CH3 groups on the same side of the double bond
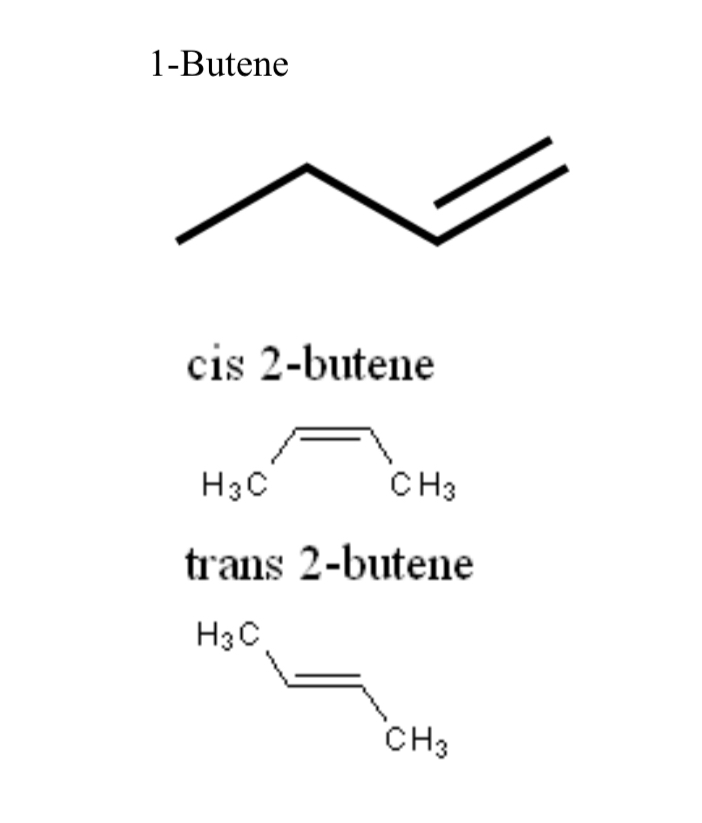
trans-2-butene
Geometric isomer of 2-butene with CH3 groups on opposite sides of the double bond