Virtual Vista science 20 Unit A chapter 3 conclusion
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
carbon-based compound
a compound primarily made up of carbon atoms
organic chemistry
the study of compounds composed of carbon
hydrocarbon
an organic molecule containing only carbon & hydrogen atoms
Alkane
a fully saturated hydrocarbon consisting of only single carbon-carbon bonds, equation: CnH2n+2 where the +2 is the end hydrogens, and the x2 because each carbon usually holds 2 hydrogen in a chain.
Alkene
A hydrocarbon with at least one carbon double bond
equation: CnH2n where the lack of +2 is due to the fact two carbons share 2 electrons thus two hydrogen are remove from the equation, balancing -2 and +2 hydrogen. An electron bond takes up one spot in both valence shells, and does the work of two hydrogen.
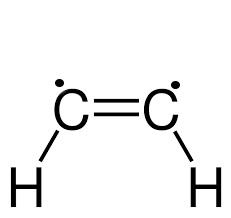
Alkyne
A hydrocarbon with at least one carbon triple bond
equation: CnH2n-2 where -2 is used to denote how carbons now share a total of 3 electrons, leaving room only for one more bond for each electron. The one electron each the two hydrogens provided to the double bond are now no longer needed, as the carbons get it from each other.
continuous-chain alkane
an alkane consisting of one simple chain of carbon atoms
complete structural
diagram that uses a line to convey bonds derived from electron sharing
..h. h. h h
…|. |. |. |
h-c-c-c-c-h or c=c etc
…|. |. |. |
…h h h h
condensed structural
diagram that uses a line for carbon-carbon bonds and includes the chemical formula for carbon hydrogen bonds.
CH3 -CH2 etc
line structural
diagram that uses only lines for carbon-carbon bonds, where you assume the hydrogen is attached to the carbon
prefix
first syllable, in hydrocarbons it indicates # of carbons.
suffix
second syllable, indicates family (alkane has the suffix ‘-ane’)
branched alkane
Alkanes that have branches which extend from them.
alkyl group
An alkane branch of a larger molecule with one hydrogen removed. I.e the branch
What is the maximum amount of bonds a carbon atom can have?
With 4 empty spots in the valence shell, carbon can share up to 4 electrons with other atom(s). Carbon has a wide range of possible combinations with this configuration.
What is the significance of hydrogen and by association hydrocarbons?
Hydrogen only needs 1 extra electron to fill its valence shell, therefore it can easily chain with carbon.
What suffix do alkyl groups (branches) use?
The suffix: ‘-yl’. The prefix are the same as continuous chain prefixes, but with the ‘-yl’ suffix. (meth-yl, eth-yl instead of methane, ethane)
What are the steps for naming hydrocarbons?
find the longest chain (may not be in a straight line)
find all the branches & name
number the parent chain starting from the end nearest to a branch
organize branch numbers, if there are 2 methyl branches, the carbon is ‘dimethyl’
alphabetize, ignore prefixes (di, tri, tetra, etc). E is before M so ‘ethyl’ goes first.
A hydrocarbon with the longest chain having 7 carbon atoms (hept)
2 branches consisting of 1 carbon (di-meth)
1 branch consisting of 2 carbons (eth)
Counted from the end closet to a branch, the methyl are attached to carbon 2 and 3, and the ethyl to carbon 4 of the longest branch. Therefore:
4-ethyl-2, 3-dimethylheptane
saturated hydrocarbon
a hydrocarbon only containing single covalent bonds between carbons (another name for Alkane)
unsaturated hydrocarbon
a hydrocarbon with double/triple covalent bonds between carbons.
Fatty acid
organic molecule; chain of carbons with one COOH group at the end and a methyl group at the other. Builds up molecules of fat.
.h
methyl : h-c-
.h
monounsaturated fat
a fat molecule that includes fatty acids which only have one double bond.
polyunsaturated fat
a fat molecular compound that includes fatty acids which have more than one double bond
essential fatty acid
a fatty acid the body cannot make itself and must get from outside sources.
hydrogenation
process that inserts hydrogen, turning unsaturated double & triple bonds into saturated single bonds
industrially produced trans fatty acid
synthetic molecule that has hydrogen atoms on opposite sides of the bonded carbon, resulting in a straighter chain
industrially produced trans fat
a fat molecule with at least one trans fatty acid chain. Usually made with partial hydrogenation
dietary cholesterol
substance found in animals as food
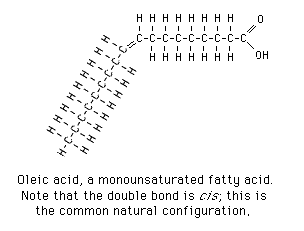
How does margarine, derived from a molecule with weaker attraction, maintain a solid state at room temp?
Normally, oleic acid, because of the bend placing the molecules farther away (not able to pack as closely together), have weaker attraction therefore less energy is needed to separate them.
However, through partial hydrogenation, hydrogen is placed on either end, balancing the molecule and making it straighter, therefore the atoms pack together closer, contributing to a stronger attracting force. More energy is required to separate the bonds raising the melting and boiling point.
trans means “on opposite sides” in latin, which is why these types of molecules are called trans fatty acids.
cis means “on the same side” in latin
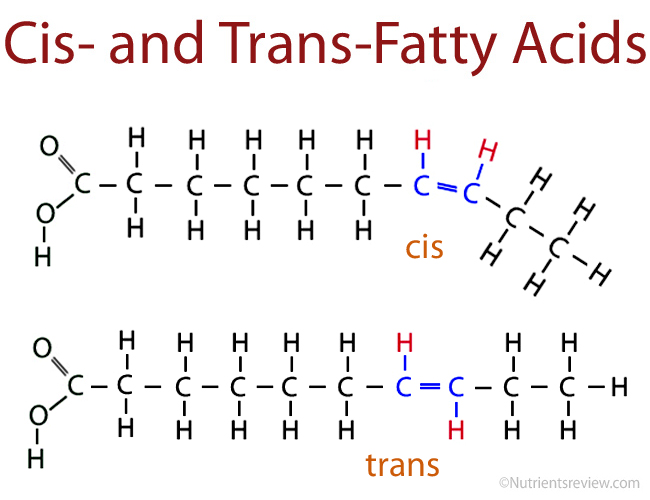
boiling
Transition from solid to gas
melting
Transition from solid to liquid
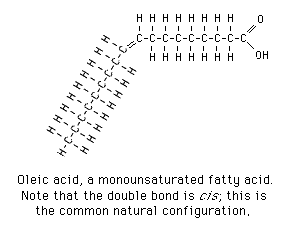
Why is this molecule called ‘omega-9’?
take note of the end with a methyl hydrogen group, and the end with a COOH group. Counting from the omega group— methyl— the bend (double bond) comes after the 9th carbon, hence it is an ‘omega-9’ fatty acid.
Alkene and Alkyne suffix’s?
Note: the longest chain must be made to include the bond
Alkene: ‘-ene’
Alkyne: ‘-yne’
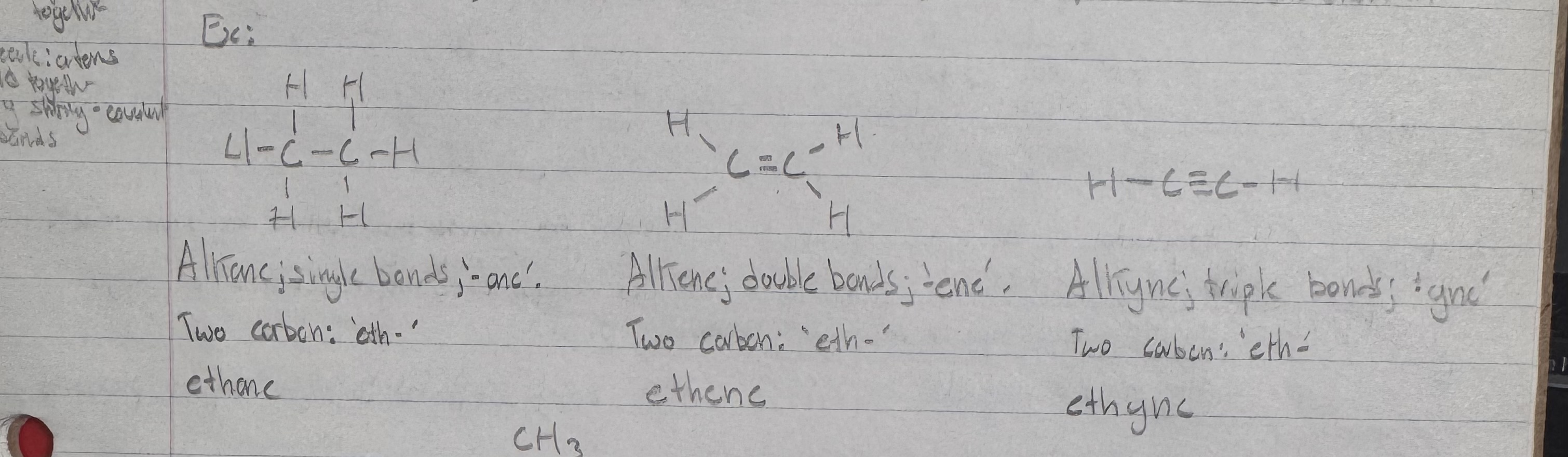
Steps to naming bonded carbons?
parent chains must include bonded carbons
count starts from the bonded carbon, the bond must happen after the lowest carbon

why do boiling and melting points increase with amount of atoms?
Larger molecules often have greater mass and more surface area, leading to stronger and more numerous intermolecular forces. As a result, more energy is required to break apart a molecular compound.
What is the difference in volatility between saturated and unsaturated hydrocarbons?
unsaturated hydrocarbons (more than one bond between atoms) are more volatile (reactive) than saturated hydrocarbons (single bonds.
Process of hydrogenation
first, the unsaturated bonds are broken, then hydrogen is inputted into the molecule. This leads to a saturated single bond hydrocarbon.
petroleum
liquid mixture of hydrocarbons formed over millions of years from the remains of ancient microscopic marine organisms.
Petroleum fractions
Petroleum consists of groups of different length hydrocarbons, and can be separated into these group lengths, which are fractions of the total mixture.
refining
an industrial process that separates, purifies, and alters raw materials.
Fractional distillation
The petroleum “soup” is first vaporized, separating the different fractions based on their boiling points. Inside a column heated from the bottom, the vaporized fractions rise. As they move up the column, the temperature gradually decreases. When a fraction cools to a temperature slightly below its boiling point, it condenses and turns back into a liquid, allowing it to be collected. This happens because the temperature is no longer high enough to maintain the vapor state, and its too cool for the fraction to become a gas, ergo it collects as a liquid.
The lighter fractions may have a boiling point at say, room temperature, so they maintain a gas state as they rise and are collected as gasses.
Naptha
A long mixture of hydrocarbons between 5 and 10 carbon atoms, which is dense enough to collect at the bottom of column as liquid.
Cracking
breaking down leftover longer chains into short molecules. There isn’t a set way these are broken down, and can be broken down into alkene and alkyne molecules.
C12H26dodaecane —> C8H18octane + C4H8butene
If n = 4, and H=8, it must be an alkene as its formula is CnH2n. Also, n=4 means four carbons, so ‘but-’ prefix.
other examples:
C12H26dodaecane —> C10H22decane + C2H4ethene
C12H26dodaecane —> C4H8butene + C5H12pentane + C3H6propene
The two types of cracking?
Thermal cracking: high temp and pressure. 750o c heat, 70 atm
Catalytic cracking: low temp and pressure. Passed through a zeolite catalyst which tends to break the bonds, 500o c
Petrochemical
a chemical made from petroleum
Combustion
a rapid reaction with oxygen that produces energy and oxides
polymerization
a reaction where short hydrocarbons join together to form longer hydrocarbon chains
polymer
the result of polymerization; a larger hydrocarbon molecule
What is the use of hydrocarbons after refining?
combusted/burned for energy
raw material for making products
Why are hydrocarbons excellent fuels?
relatively stable & easily transported
have bonds that store a lot of energy
readily available
combustion
non-spontaneous reaction, requiring external energy to break bonds. The atoms recombine in different configurations afterwards. INCLUDE PIC
also: a rapid reaction with oxygen that produces energy and oxides