Reactions of Acids and Bases
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
What are the 3 MAIN acid reactions
Acid + metal —> Salt + Hydrogen gas
Acid + Metal Oxide —> Salt + Water
Acid + Salt of Weak acid —> Salt + water + CO2
** All these rxn are neutralization rxn, making them exothermic.
2
New cards
To find equation: find T.I.E. then N.I.E.
TO find T.I.E.: break up all aqueous substants into their ions
N.I.E cancel all spectaor ions, which are ions that appear on both sides of the equation
3
New cards
What are Antancids
Used to alleviate excess HCL in stomach (heartburn)
Antacids are weak bases which neutralize excess stomach acid
Produces CO2 causes burping
4
New cards
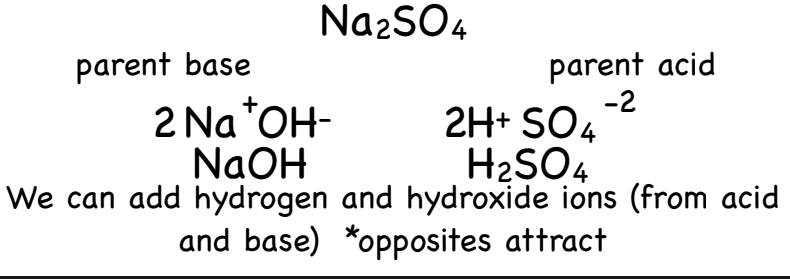
How to identify parent acids and bases?
Take the salt and split it into a cation and an anion
Example Image: