Biological Molecules 2.1.2
1/88
Earn XP
Description and Tags
Not finished. Need points ,g,h
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
Water with hydrogen bonds image
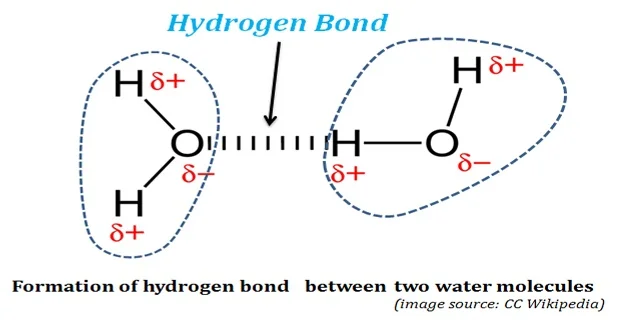
What is it called when one side is positive and the other side is negative
Di-pole effect, oxygen = negaitve
Describe the structure of a water mol.
Water is a polar molecule
Hydrogen bonds form when a slightly negative oxygen of one molecule comes close to a slightly positively charged hydrogen on another molecule
How would you get 3 marks for describe the structure of a water mol. without writing the whole thing out
DRAW IT
Label the hydrogen bonds and the delta -/+
Draw and and label h+ bond
WRITE water is a polar molecule
What are the properties of water
Has a high boiling point
Ice is less dense than water
Cohesion
Adhesion
Water acts as a solvent
Transport medium
Evaporates
High specific heat capacity
High latent heat of vaporisation
Capillary action
Why is a high boiling point of water important
A lot of energy is needed to break the many hydrogen bonds
Examples of water with a high boiling point in nature
Stable water temperature for aquatic animals
Less energy spent on temp control
Why is ice being less dense than water important
Creates an insulating barrier
Water below doesn’t freeze
Allowing organisms to move under water
Examples of ice being less dense than water in nature
Habitat for animals like polar bears on top of ice
Fish can move under the water
Why is cohesion in water important
Cohesion = hydrogen bonding in water
Examples of cohesion in nature
Creates high surface tension for insects like water skaters to walk on
Water moving through the xylem
Why is adhesion in water important
Adhesion = waters attraction to other molecules/ surfaces
Examples of adhesion in nature
Water moving through the xylem
Why is water acting as a solvent important
Allows mineral ions to be transported around plants and animals
Example of water acting as a solvent in nature
Substances dissolving in the blood stream
Why is water being a transport medium important
Allows transport of soluble substances
Example of water being a transport medium in nature
Transporting substances around the body in the blood or around a plant
Why is evaporation of water important
Evaporation is a cooling mechanisms - the evaporating water takes heat away from the body
Example of evaporation of water in nature
Sweating in mammals or panting in dogs to cool them down
Why is high specific heat capacity of water important
Takes a lot of energy to heat up water by one degree
Example of high SHC of water in nature
A stable temperature
Enzyme can work at optimum temp
Gases remain soluble for aquatic organisms
Why is high latent heat of vaporisation of water important
Takes a lot of energy to change water from a liquid to a gas form
Example of high LHV of water in nature
Metabolic reations
Why is capillary action of water important
Allows water to move up narrow vessels
Example of capillary action of water in nature
Water moving up the xylem in plants
Condensation reaction
Covalent bond formed
Water mol. is removed
Hydrolysis reaction
(hyBROlysis)(hydro flask = water in the bottle)
Covalent bond broken
Water mol. is added
Elements in carbohydrates
Carbon
Hydrogen
Oxygen
Elements in lipids
Carbon
Hydrogen
Oxygen
Elements in proteins
Carbon
Hydrogen
Oxygen
Nitrogen
Sulfur
Elements in nucleic acids
Carbon
Hydrogen
Oxygen
Nitrogen
Phosphate
What is a monomer (General definition)
Single unit
What is a polymer (General definition)
Several monomers joined together
Monosaccharides
One unit of sugar
Simple sugars that form the monomer units
Disaccharides
Two monosaccharide mol. joined together
Polysaccharide
More than two monosaccharides joined together
e.g Starch
Two main monomers with carbohydrates
Alpha and Beta glucose
Alpha glucose diagram
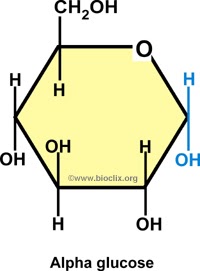
NEEDS NUMBERS
Beta glucose diagram
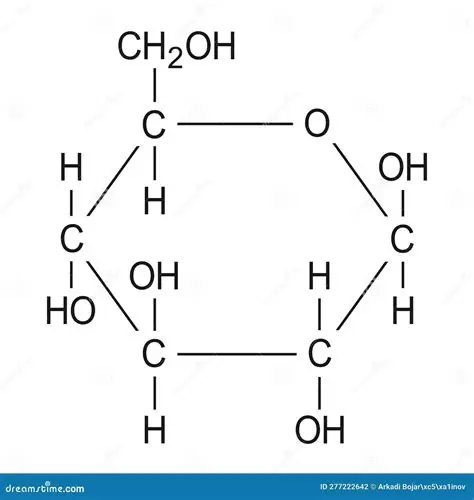
NEEDS NUMBERS
Key difference between alpha and beta glucose
In alpha glucose - carbon 1, H is above while the OH is below
In beta glucose - carbon 1, OH is above and the H is below
Way to remember the difference between Alpha and Beta glucose
Glucose being a hexose sugar definition
6 carbon atoms
Glucose being reducing sugar meaning
When its in solution it can reduce other chemicals
Pentose sugar meaning and example
Has 5 carbons
Ribose - C5H10O5
Found in RNA
Disaccharide bond image (alpha)
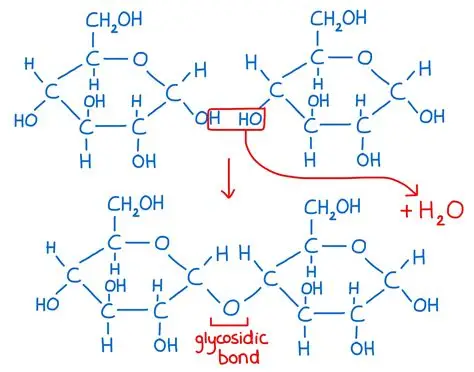
Alpha glycosidic bond between Carbon 1- 4
Covalent bond in carbohydrates
Glycosidic bond
When beta glucose bond
Orientate itself 180 degrees
Disaccharide bond image (beta)
…
Maltose
α-glucose and α-glucose
Lactose
β-glucose and galactose
Sucrose
α-glucose and fructose
Where is starch found and what is it made of
Plants
Mixture of amylose and amylopectin
Amylose structure and properties
alpha 1-4 glycosidic bonds
Coiled like structure
Compact
Doesn’t affect osmotic potential
Good for storage
Amylopectin structure and properties
alpha 1-4 glycosidic bond
alpha 1-6 glycosidic bond
= branches
Allows hydrolysis at the ends by enzymes to create monosaccharides
= available for aerobic respiration
Glycogen structure and properties
alpha 1-4 glycosidic bond
alpha 1-6 glycosidic bond
wont affect osmotic potential
compact
lost of ends to be hydrolysed to create alpha glucose = available for respiration
Similarities and differences between starch and glycogen
Made of alpha glucose
Storage mol. of carbohydrates
Similar makeup and properties
Starch = plant
Glycogen = mammal livers
Cellulose
Beta glucose - orientate itself 180 degrees
Beta 1-4 glycosidic bonds
No osmotic potential
Inert (unreactive)
Hydrogen bonds form btw. chains of cellulose = high TENSILE strength
Crosslinks btw. the microfibrils (each chain)
More in between - after thsi point starts at spec pint h, i j
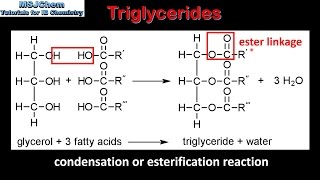
Structure of a triglyceride (image)
Structure of a triglyceride (written)
3 (hydrophobic) fatty acid (tails)
1 glycerol
What bond forms between fatty acids and glycerol
Ester bonds
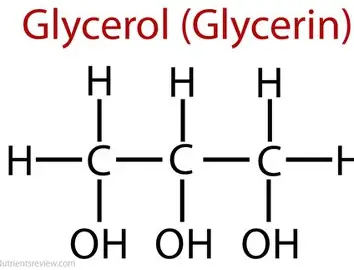
What is the structure of glycerol (image + words)
C3 H8 O3
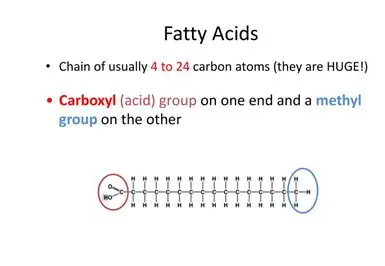
What is the structure of fatty acid chains (image + words)
Hydrocarbon chains
How do fatty acids and glycerol bind
Glycerol binds to the fatty acid (on carboxyl end) and water is removed (condensation reaction)
2 types of fatty acid chains
Saturated
Unsaturated
What the difference between a saturated and a non saturated fatty acid chain
Saturated, has no double bonds (every carbon is fully saturated with the hydrogen mol.)
Image of saturated and non saturated fatty acid chains
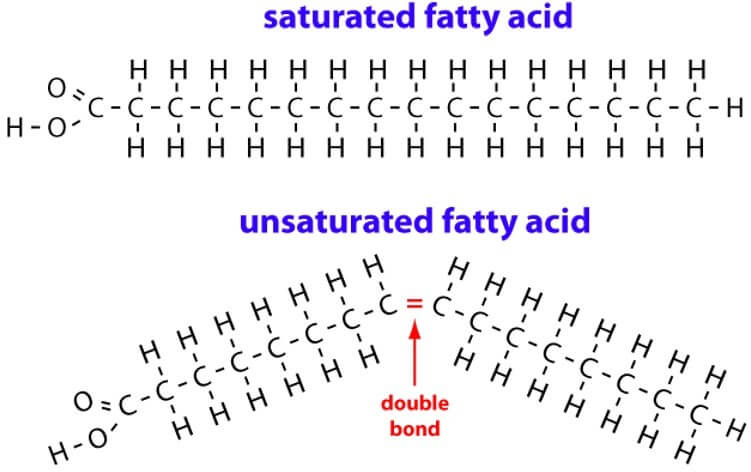
What different property does the saturated fatty acid chain make
Double carbon carbon bond = kink in chain = bend
= not as close = less dense substances such as oil
(saturated fatty acids = solid fatty substances)
Phospholipid structure (image)
Phospholipid structure (written)
two fatty acid tails
a glycerol molecule
a phosphate head
Properties of the phospholipid
Fatty acids = non polar and insoluble in water - hydrophobic
Phosphate group = polar and water soluble - hydrophilic, it orientates towards water
What is the purpose of phospholipids
Phospholipid bilayer which forms membranes
Bilayer - water on outside of cell and water on inside of cell
What structure would it form if water was everywhere around the phospholipid bilayer
Micelle structure
Micelle structure image
Triglyceride function
Good at storing energy
Respiratory substrate - release water when broken down
Insoluble - doesn’t affect water potential
Used to make hormones
Water proofing
Buoyancy
Form layers of insulation
Protective layers around organs/ nerves (myelin sheath)
Aids fat absorption - fat soluble mol. e.g vit A
Cholesterol image
Cholesterol structure
4 carbon ring structure
OH group = hydrophilic
CH section = hydrophobic
Cholesterol function
Regulates fluidity
(small size and flattened shape allow them to fit between phospholipids and bind their hydrophobic tails making the membrane more rigid)
At low temps, cholesterol increases fluidity of membranes but at high temps decreases fluidity
What’s make using cholesterol
Vitim D
Steroid hormones
Bile
SPEC POINT K, L, M
Structure of an amino acid (image)
Structure of an amino acid (written)
Central C
Hydrogen
Carboxylic group (COOH)
Amine group (NH2)
R group (changes)
What happens when two amino aids join together
A dipeptide bond forms
water is removed - condensation
Peptide bond image
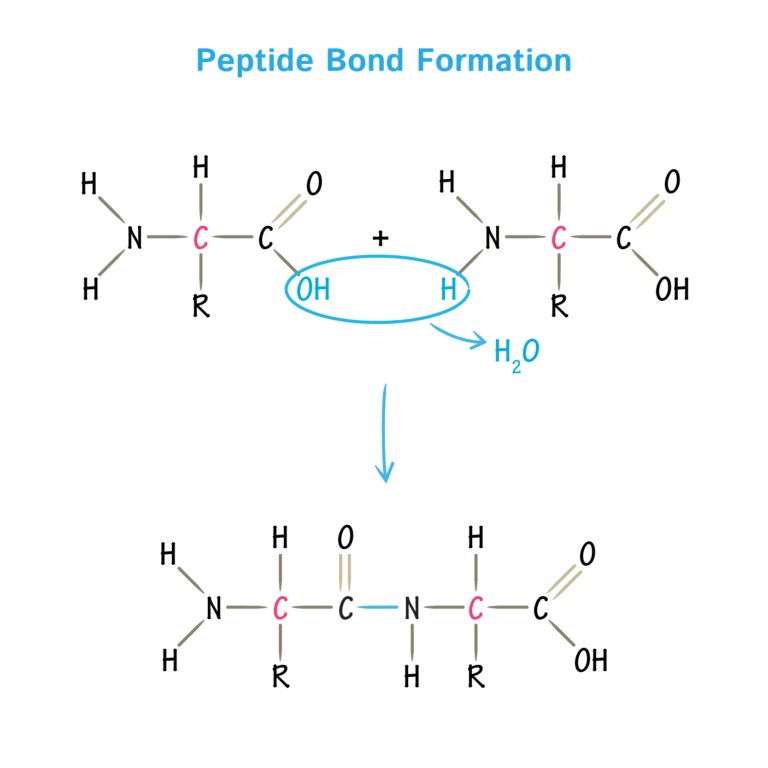
What is the primary structure
The sequence of amino acids bonded by peptide bonds
Polypeptide
What is the secondary structure
Folding of the polypeptide chain
Held in place with hydrogen bonds between the r groups
(Hydrogen bonds form btw. slightly positive hydrogen and slightly negative oxygen)
Alpha helix or beta pleated sheets
What is the tertiary structure
Further folding of the polypeptide chains
Held by hydrogen bonds ( delta + Hydrogen and delta - oxygen)
Disulphide bridges (btw. 2 sulphers)
Ionic bonds (positive and negative r groups)
Hydrophobic R groups (towards center of protein)
Hydrophilic R groups (towards outside of protein)
What is the quaternary structure
More than 1 polypeptide chain