BIOL 4102 Midterm Study Notes
1/163
Earn XP
Description and Tags
Generalized Assortment of Information for Studying for BIOL 4102 Midterm According to learning Objectives and self selected pieces of information.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
164 Terms
What is the difference between General Pathology and Systemic Pathology?
General Pathology is the study of REACTIONS of CELLS to TISSUES and pathological stimuli
Systemic Pathology is the study of reaction and abnormalities in organs
Both are required when assessing pathology
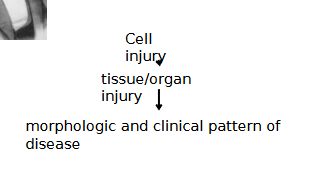
What are the stages in the development of disease?
They are in order…
1. Etiology (Cause of disease)
2. Pathogenesis (Mechanism of disease)
3. Abnormalities in cells and tissues (be they molecular, functional or morphological)
4. Clinical Manifestations (signs and symptoms)
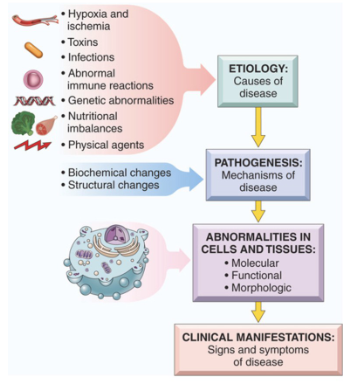
Cellular Responses To Stress & Noxious Stimuli Included what 4 criteria?
A. Causes of Cell Injury
B. Cell Injury and Cell Death (*Reversible and Irreversible)
C. Mechanisms of Cellular Injury and Death
D. Cellular Adaptations to Stress
What are some of the causes of Cell Injury listed from very common to very uncommon?
Oxygen Deprivation in the form of Hypoxia and Ischemia
Toxins (Such as drugs and other environmental agents)
Infectious agents (Bacteria, viruses, fungi, parasites)
Immunologic reactions (Allergic reactions and autoimmune reactions)
Genetic abnormalities (aa substitution, protein misfolding)
Nutritional imbalances (malnourishment vs over consumption)
Physical agents (temperature, trauma, radiation, electrical shock)
Aging
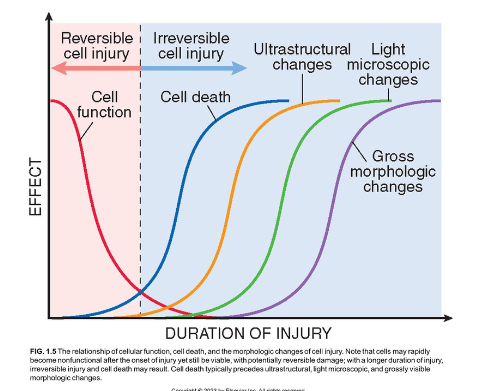
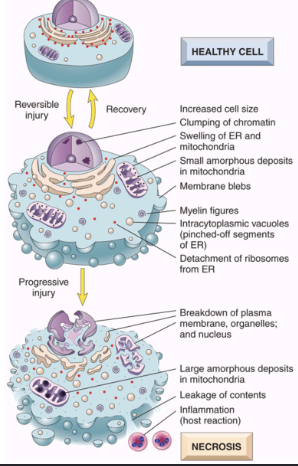
At what is that relationship between reversible and irreversible cell injury with morphology and function?
Reversible cell injuries tend to preserve cell function more so that irreversible cell injuries. In this similar vein, they are also more morphologically observable, requiring less tools to asses. On the contrary, Irreversible cell function can can lead more towards cell death as the morphological changes become less apparent to more apparent as the duration of the injury increases.
What are the Two Main types of Reversible Cell Injury?
1. Cellular Swelling
Failure of ion pumps in membrane to maintain ionic and fluid balance, leading to possible lysis and endosmosis occurs resulting from cell presence in hypotonic solution
2. Fatty change (Steatosis)
form of Hypoxic Injury, although can have various metabolic and toxic injury types
contains lipid vacuoles in cytoplasm
common in cells involved in lipid metabolism such as hepatocytes or myocardial cells
In the Proximal Rabbit Kidney Tubule Example, what caused the loss of microvilli and blebbing of the membrane?
Reperfusion following ischemia
What are some other morphological changes seen in cell injury?
Eosinophilia in cytoplasm which gets emphasized more in necrotic cells.
Myelin figures, which are collections of phospholipids resembling myelin sheaths that are derived from damaged cells can be found in both irreversible and reversible cell injuries.
What is Necrosis?
Necrosis is when a severe disturbance or action of a toxin causes a quick and uncontrollable cell death.
Often called “accidental cell death”
What happens during necrosis?
Loss of membrane integrity
Leaking of cellular contents → enzymatic digestion of organelles & cytosolic components
Increased eosinophilia via ↓ cytoplasmic RNA
Myelin figures
Patterns of nuclear changes (due to breakdown of DNA & Chromatin)*
Inflammation (at late stages)
Cellular Swelling
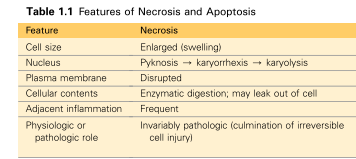
What are the Three Patterns of nuclear changes that can occur during Necrosis*
Pyknosis - Nucleus shrinks and chromatin clumps, making it highly basophilic
↓
Karyorrhexis - Chromatin dissolution due to action of DNAses and RNAses = Pyknosis nucleus fragments
↓
Karyolysis - Fading of basophilia (Loss of DNA due to endonucleases)
What are the Five different patterns of tissue injury related necrosis?
Coagulative Necrosis
Liquefactive Necrosis
Caseous Necrosis
Fat Necrosis
Fibrinoid Necrosis
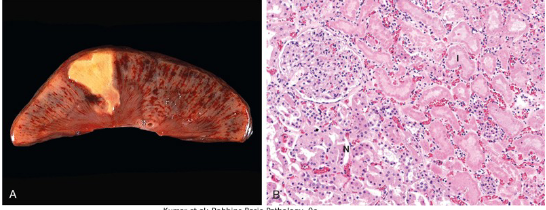
What is Coagulative Necrosis?
Coagulative Necrosis is a common type of necrosis that is known for its presence of infarcts (area of ischemic death) that is present in all solid organs EXCEPT the brain.
It has architecture of dead tissue that is preserved.
Protein denaturation PREDOMINATES over heterolysis or autolysis
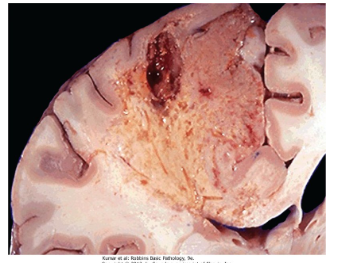
What is Liquefactive Necrosis?
Liquefactive Necrosis is a type of necrosis that is often seen at sites of bacterial or fungal infections that result due to an accumulation of inflammatory cells (leukocytes, among others) and enzymes that quite literally “liquefy” them.
Hypoxic Cellular Death in CNS is a good example of this
This results in the formation of abscesses which are creamy yellow pus spots where dead cells are completely digested.
Is where Heterolysis or Autolysis PREDOMINATES over protein denaturation
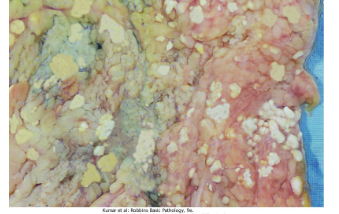
What is Caseous Necrosis?
Caseous Necrosis is a type of necrosis which in some ways can be thought of as an furtherance of coagulative necrosis, with the distinct trait that tissue architecture and cellular outlines are not able to be discerned.
This necrosis is characteristic for its foci of tuberculosis infection, forming a friable yellow-white “cheese-like” appearance on the surface of the lung.
Often associated with granuloma (will get into more later)
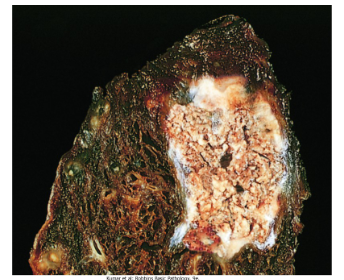
What is Fat Necrosis?
Fat Necrosis is a focal area of fat destruction seen in cases of abdominal trauma or acute pancreatitis.
It is caused by lipase activation from the pancreas, which happens when enzymes leak out of the damaged pancreatic acinar cells and ducts and begin to digest fat cells and their contents including triglycerides.
The fat combined with calcium results in the formation of white soapy deposits
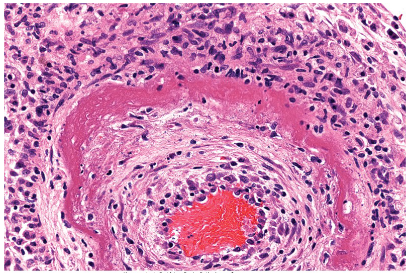
What is Fibrinoid Necrosis
Fibrinoid Necrosis is a special form of necrosis that can only be seen in a light microscopy.
It may result when complex antigens and antibodies are deposited in the walls of blood vessels and in severe hypertension.
Deposited immune complex and plasma proteins that have leaked into the wall of injured vessels produces Bright Pink amorphous ring called a fibrinoid.
Often seen in cases of vasculitis and in transplanted organs undergoing rejection.
Continue with Apoptosis
Continue On Slide 18 and textbook page 23
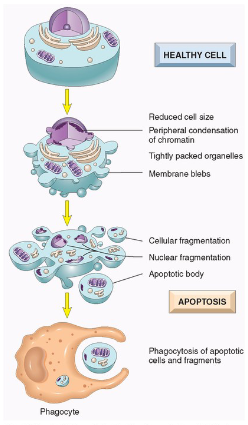
What is Apoptosis?
Apoptosis is.
Regulated Cell Death
Involves Enzymatic Degradation
Nuclear DNA & proteins
Cytoplasmic proteins
Forms cytoplasmic blebs and apoptotic bodies
Apoptotic bodies targeted for phagocytosis
No Inflammation
Can coexist with necrosis
What are some important Pathological Causes of Apoptosis?
DNA Damage
Accumulation of misfolded proteins
Infections, especially Viral Infections
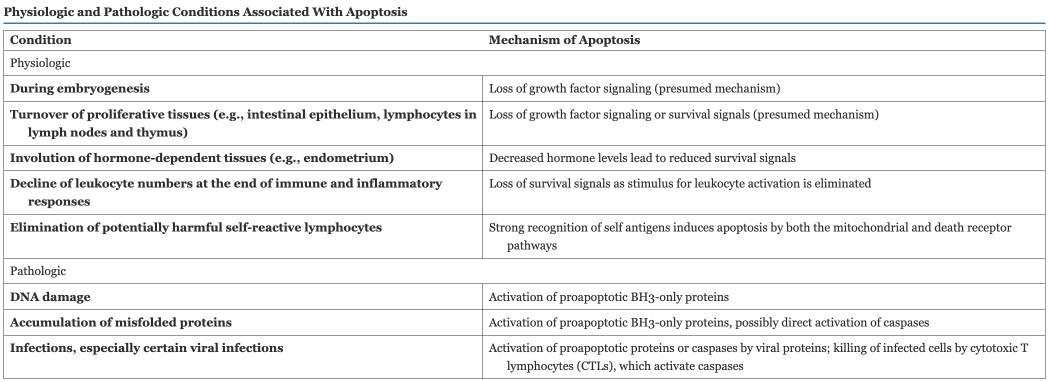
Cell Survival and Apoptosis Pathways
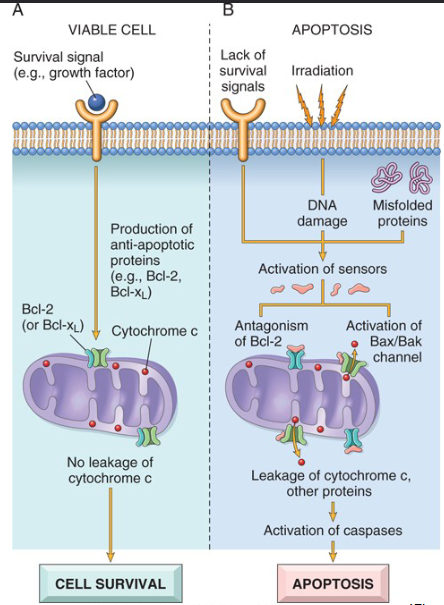
What are the Two Major Mechanisms of Apoptosis
Mitochondrial (Intrinsic Pathway)
Cell survival vs. death is determined by membrane permeability of mitochondria
Controlled by more than 20 proteins (Bcl-2 Family)
Bcl-2 and Bcl-x are anti apoptopic while Bax and Bak are pro apoptotic
Death receptor (Extrinsic Pathway)
Death receptor expressed on cell surface can trigger apoptosis
tumor necrosis factor (TNF) receptor family
cytoplasmic regions contain a conserved “death domain”
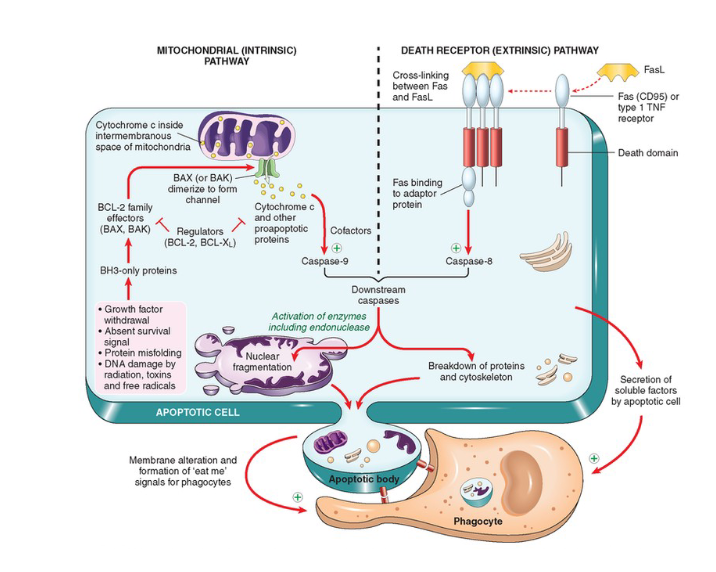
Close Up On Death Receptor Pathway
In Example Checkpoint, TNFa acts as ligand for a TNF receptor similar to how FasL acts as a receptor to Fas (CD95) or Type 1 TNF receptors
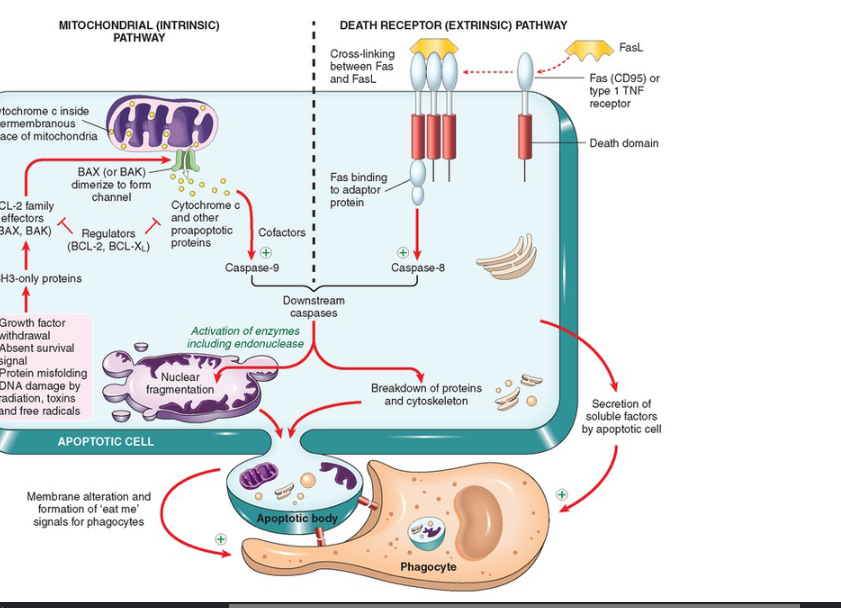
Death Domain Explained
What is TP53 and why is it called the guardian of the genome?
The TP53 gene encodes p53, a crucial tumor suppressor protein often referred to as the "guardian of the genome." This designation stems from its pivotal role in preserving genomic stability by preventing the accumulation of mutations that can lead to cancer. TP53 is one of the most frequently mutated genes in human malignancies, emphasizing its importance in cell cycle regulation, DNA repair, and apoptosis.
What does FLIP do to caspase stimulation?
FLIP works as a caspase antagonist, blocking activation of downstream effects of death receptors
These are often produced by Viruses in order to keep infected cells alive.
What type of chemical is responsible for the clearance of of Apoptotic material?
the “eat me” aka, phosphatidylserine that signals phagocytic cells
Is present on outer leaflet of cell membrane
Note: dying cells also secrete soluble factors that recruit phagocytes
What are the General Principles for the Mechanisms of cell injury and cell death?
Type, duration and severity of injury
may affect how cell respond to injurious stimulus
Type, status, adaptability & genetic make up of the injured cell
may effect degree of cellular damage. ie think of skeletal muscle vs cardiac muscle
Functional and biochemical abnormalities
may affect essential cellular components causing further injury and death
One injurious stimulus may trigger multiple mechanisms
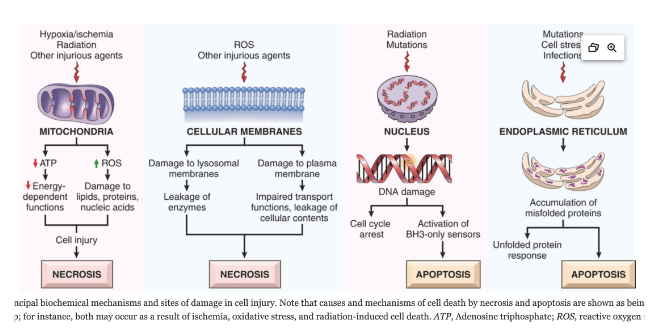
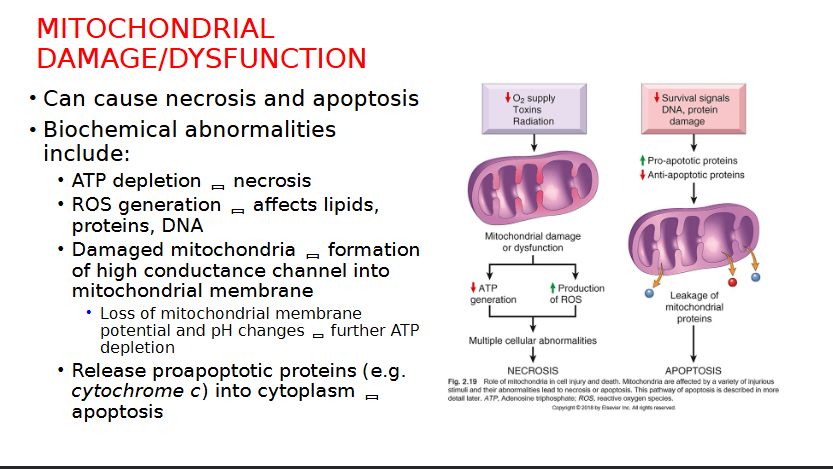
Explain Mitochondrial Damage and Dysfunction related cell injuries
Mitochondria affected by Hypoxia and ischemia
Compensated for by TF of the hypoxia-inducible factor 1 (HIF-1) Family
Synthesis of proteins to help with cell survival in low oxygen
Effect ATP Generation leading to ATP depletion, disrupting membrane transport, protein synthesis and lipogenesis
Ischemia can cause necrosis by…
Note: Injures tissues faster than hypoxia
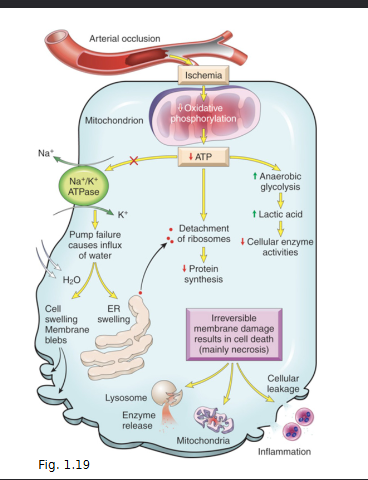
What are the two mechanism by which a reperfusion injury can take place after an Ischemic episode
Increased Generation of ROS due to …
mitochondrial damage
Loss of antioxidant defence mechanism
leukocyte infiltration
Inflammation leading to tissue injury
increased influx of leukocytes and plasma proteins (chapter 2)
increases release of products of activated leukocytes
complement activation and by-products
How is Oxidative Stress related to cell Injury
Oxidative stress refers to cellular damage induced by accumulation of ROS, in the form of free radicals.
Involved in cell injuries such as:
hypoxia
ischemia reperfusion
chemical and radiation
tissue injury via inflammatory cells
cellular aging
may cause necrosis, apoptosis, or necroptosis
What are the two major pathways of ROS production?
Normal Redox reaction in mitochondrial metabolism
Respiratory burst in phagocytic cells
Free Radicals involved in Cell Injury
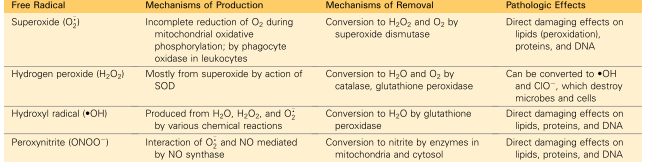
What are the systems used to remove free radicals from a cell?
Superoxide dismutase in the mitochondria and cytosol
glutathione peroxidase in the mitochondria and cytosol
Catalase in peroxisomes
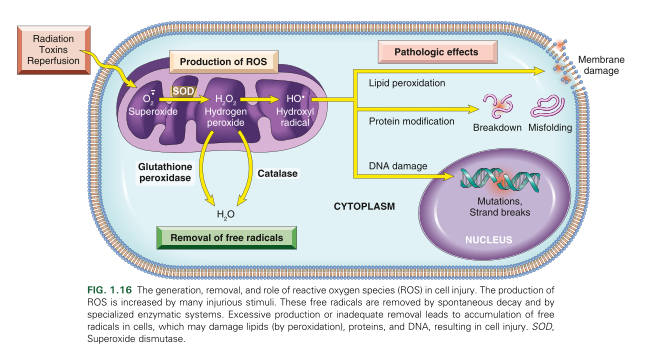
That are the pathological effects of ROS?
Lipid peroxidation of membranes
DB are susceptible to attack by ROS leading to unstable peroxides and unstable membrane
Protein modifications
promote sulfhydryl induced protein crosslinking which enhances degradation or loss of enzyme activity
Direct effect on polypeptide fragmentation leading to protein misfolding
DNA Damage
ROS binds to thymine which targets and breaks single stranded mitochondrial and nuclear DNA
Implicated in apoptosis cell death
How is Chemical Injury related to Cell injury and cell death?
Directly and Indirectly
Directly due to chemicals binding to critical molecular components, such as the cases of mercury and anticancer drugs.
Indirectly due to biologically inactive chemicals being converted to reactive toxic metabolites and the function of cytochrome p-450 mixed oxidases
metabolites might cause membrane damage and cell injury by bind to proteins and lipids they shouldn’t
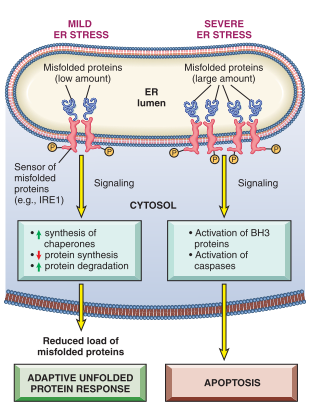
How does ER Stress relate to cell injury and cell death?
Accumulation of misfolded proteins in cells leaking to ER Stress
This causes an increased production of misfolded proteins
such happens in aging and genetic mutations
Reduced ability to eliminate misfolded proteins
Induce “unfolded protein response” as a protective mechanism, causing an increased production of chaperones and decreases protein synthesis
Apoptosis by mitochondrial (Intrinsic) pathway activated when cellular adaptive response in inadequate
activation of proapoptotic sensors via the BH-3 family
direct activation of caspases
observable in neurodegenerative disorders.
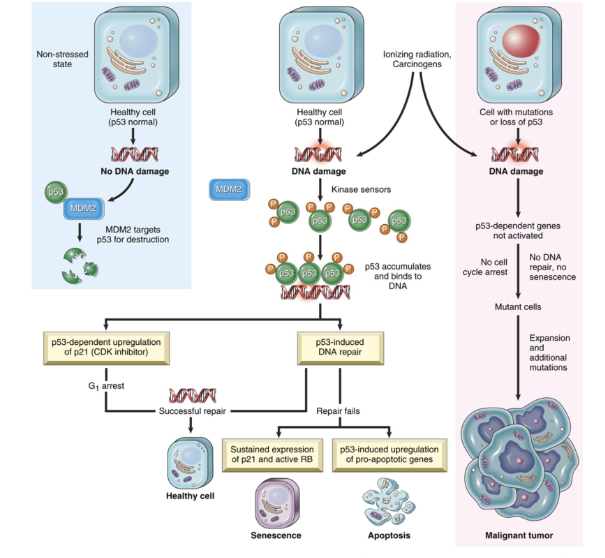
How is DNA Damage related to Cell injury and death?
Mainly being caused by Radiation, chemotherapy, ROS and mutations
Prevents cells from becoming a tumor if the p53 gene is able to act functionally, as MDM2 will targets p53 for destruction
Repair via p53 also possible, as p53 arrests the cell cycle at G1 to allow for repair
Mitochondrial Damage and Dysfunction
Defects in Membrane Permeability
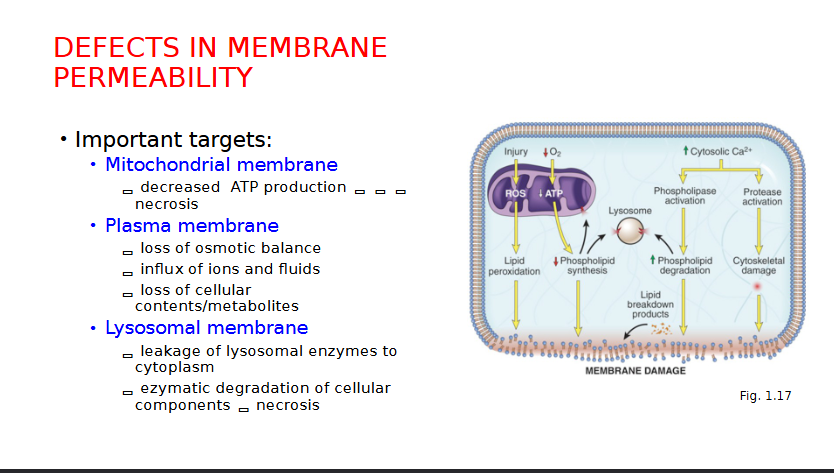
Cellular Adaptations to Stress (reversible) can cause changes to cell…
Size
Number
Phenotype
Metabolic Activity
Function
Environmental Changes
What are the FOUR ways cells may respond to stress?
Hypertrophy
Hyperplasia
Atrophy
Metaplasia
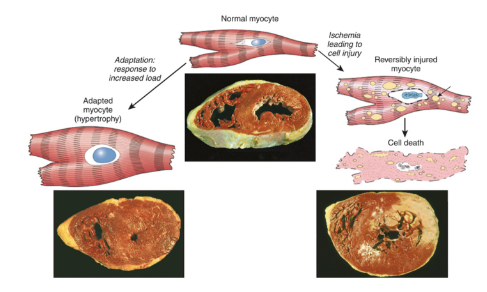
What is Hypertrophy
Increase in size of cells, leading to an increased organ size
Caused by an increase in structural proteins and organelles
Occurs when cells are UNABLE to divide
Physiological: Uterus growth during pregnancy
Pathological: Cardiac Hypertrophy due to hypertension or aortic valve disease
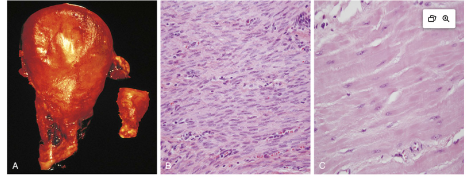
Cardiac Example Hypertrophy
protein and signalling pathway hypertrophy explanation
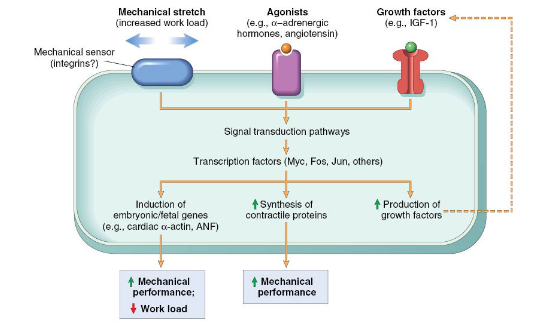
What is Hyperplasia
An increase in the number of cells leading to an increased organ size
Occurs due to proliferation and differentiation of cells
Two Physiological Forms:
Hormonal Hyperplasia
Hormones increase functional capacity when needed
increased in glandular epithelium
ie/ breast tissue during puberty
Compensatory Hyperplasia
Increase in tissue mass after damage or partial resection
ie/ Liver resection
Pathological Hyperplasia
due to excess hormones or growth factors
normal regulatory process
risk for developing cancers increases
ie/ endometrial hyperplasia and benign prostatic hyperplasia (BPH)
What is Benign Prostatic Hyperplasia?
A form of nodular pathological hyperplasia that occurs in males over 50 years old.
Etiology & Pathogenesis:
Benign proliferation of stromal and glandular elements
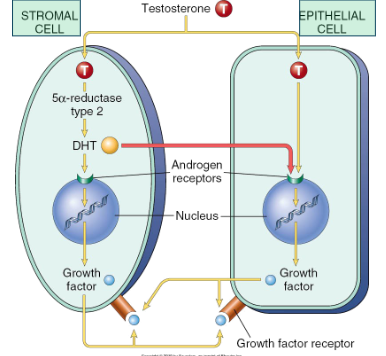
Central role of dihydrotestosterone (DHT) - androgen derived testosterone
Clinical Features:
Nocturia
Poor Urinary Stream
Acute Urinary Retention
Treatments:
α1 adrenergic receptor blockers
inhibitors of 5-α reductase
transurethral resection of prostate (TURP) and surgical alternatives
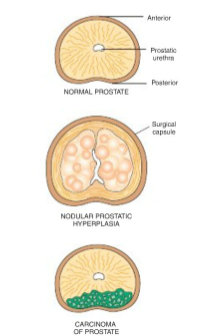
How are Stromal cells and Epithelial cells interacting with testosterone that leads to Benign Prostatic Hyperplasia?
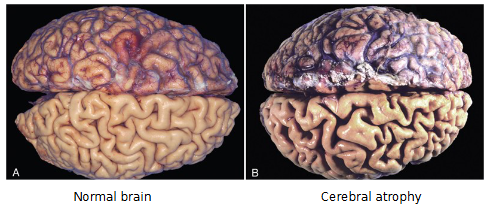
What is Atrophy?
a decrease in the size of cells, leading ot a reduction in organ size
results in decreased protein synthesis with decreased metabolic activity
increased protein degradation in cells via Ubiquitin-Proteasome pathway
Causes:
decreased workload, loss of innervation, decreased blood supply, inadequate nutrition, loss of endocrine stimulation, aging
Physiological Atrophy
atrophy of endometrium, breast and vaginal epithelium after menopause
Pathological Atrophy
decreased workload - disuse atrophy
damage to nerve supplying skeletal muscle - denervation atrophy
Diminished blood supply, ie/ cerebral atrophy
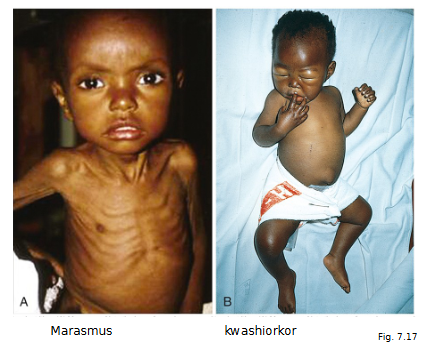
Inadequate nutrition eg. Marasmus, Kwashiorkor, Cachexia
Similarities and differences between Marasmus, kwashiorkor and cachexia
First important to understand what PEM is: Protein Energy Malnutrition
Marasmus
Insufficient calories overall (wt. < 60% normal)
Somatic protein compartment severely depleted
Pronounced muscle wasting
loss of subcutaneous fat (via cortisol-induced lipolysis)
kwashiorkor
protein deprivation relatively greater than loss in calories
visceral protein compartment (most likely liver) more severely depleted
generalized edema masks a decrease in body weight
enlarged fatty liver
Cachexia is PEM in advanced cancer or aids
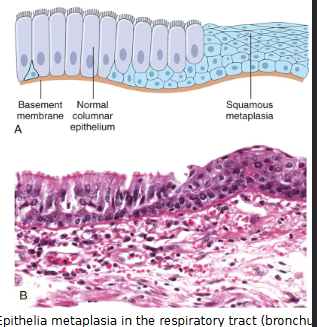
What is Metaplasia?
Reversible change from one adult cell (epithelial or mesenchymal) to another cell type.
Occurs due to reprogramming of stem cells, phenotypic change of differentiated cells by cytokines and growth factors
May initiate malignant transformation if signals for metaplastic changes persist
Most common in columnar to squamous epithelium
example. Epithelial metaplasia in respiratory tract of habitual smoker
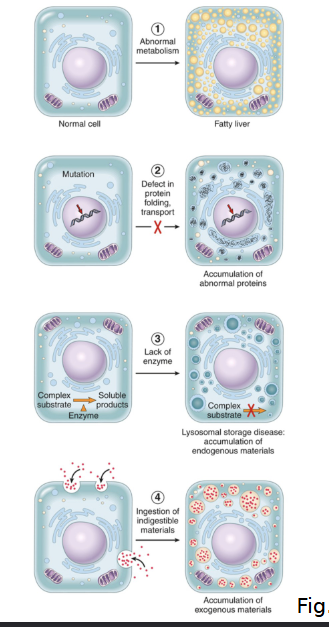
What are the FOUR main pathways that lead to abnormal intracellular accumulations?
Abnormal Metabolism ex. Fatty liver (steatosis)
Defect in protein folding/transport/secretion ex. alpha1-antitrypsin
Inherited enzyme deficiencies ex. “storage diseases”
Ingestion of indigestible materials ex/ accumulation of carbon/silica particles
What are the FOUR main types of intracellular accumulation?
Lipid
Protein
Glycogen
Pigment
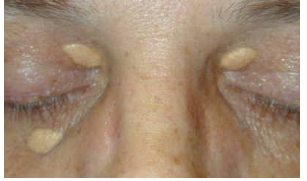
Examples of Lipid Accumulation and how its related to Intracellular Accumulation?
Examples include steatosis and Cholesterol and cholesteryl ester accumulation
Steatosis is accumulation of triglycerides in parenchymal cells that can happen at any stage be it uptake, catabolism or secretion.
Cholesterol & cholesteryl ester can be atherosclerosis where foam cells from macrophages and smooth muscle cells filled with cholesterol form
May also be xanthomas
tumorous masses of subepithelial connective tissue of skin and tendons
Examples of Protein Accumulation
Caused by excess production but not morphologically visible
Examples
Alpha 1 antitrypsin
secretory protein that inhibits proteases ie/ neutrophil elastase
Autosomal recessive disorder results in mutant proteins misfolding and polymerizing causing impairment in migration from the ER to the golgi
this disease causes low circulating levels of this protein
Mutation overall leads to pulmonary emphysema leading to lack of destructive proteases
also has hepatocellular accumulation of misfolded a1AT protein, which leads to nonfunctional proteins stores in hepatocytes that can cause apoptosis
deficiency is commonly diagnosed in infants
Reabsorption of protein droplets in nephrotic syndrome
neurofibrillary tangles in Alzheimer’s disease
How is Alpha-1 Antitrypsin Deficiency characterized in the Liver?
Globular Inclusions in Hepatocytes that ate PAS positive and diastase resistant
Periportal hepatocytes affected early and central lobular later as severity continues
Other features include hepatitis, fibrosis, to full blown cirrhosis
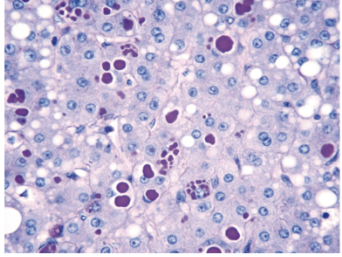
NOTE: MAKE NOTES ABOUT ASSIGNMENT 1 PRESENTATION DISEASES AS THESE MIGHT SHOW UP ON THE MIDTERM
What is Glycogen Accumulation
Excess glycogen deposit associated with abnormal metabolism of glucose and glycogen
Common in Diabetes mellitus when its poorly controlled, resulting in abnormal glucose metabolism and accumulation in the renal tubular epithelia, cardiac myocytes and beta cells of the islets of langerhans
What is Pigment Accumulation and what are the two types?
Exogenous:
Carbon and coal dust causing anthracosis
Endogenous:
Melanin causing Brown black pigmentation of skin
Freckles for instance
help protect skin against harmful UV radiation
Other examples could include Lipofuscin and Hemosiderin
What is Lipofuscin
A pigment that is found as a marker of past free radical injury and lipid peroxidation
called a wear and tear pigment
In liver and brain
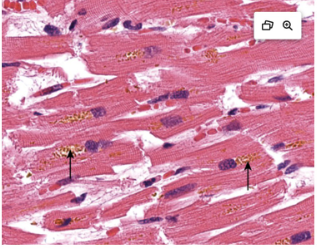
What is Hemosiderin
Hb derived, golden yellow to brown pigment
Ferritin micelles form hemosiderin granules
Accumulates due to local excess (such as a hemorrhage)
Or
Systemic excess (Such as hemolytic anemia, hereditary hemochromatosis)
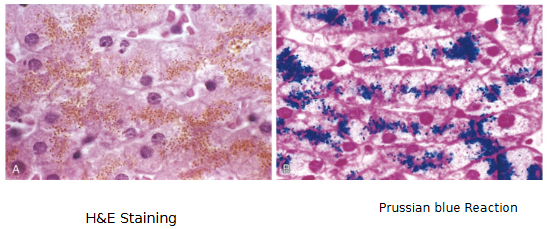
What Pathologic Calcification
Common process in disease states
Abnormal depositions of calcium salts
can be two forms
In DEAD and DYING CELLS = Dystrophic
In normal tissues = Metastatic
Dystrophic Calcification
Normal calcium metabolism but it deposits in injured or dead tissue (e.g. areas of necrosis)
Arterial lesions of atherosclerosis
May cause organ dysfunction
Important cause of aortic stenosis in the elderly
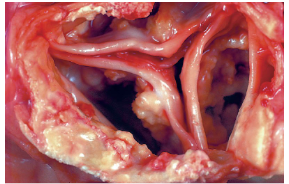
Metastatic Calcification
In normal tissue during hypercalcemia
• Affects the tissues of the vasculature, kidneys, lungs
and gastric mucosa
• Causes:
Increased PTH secretion (hyperparathyroidism, PTH
secreting malignancy)
Bone destruction (tumors in bone, Paget disease)
Vit D related disorders (vit D intoxication, sarcoidosis)
Renal failure
• Phosphate retention = secondary hyperparathyroidism
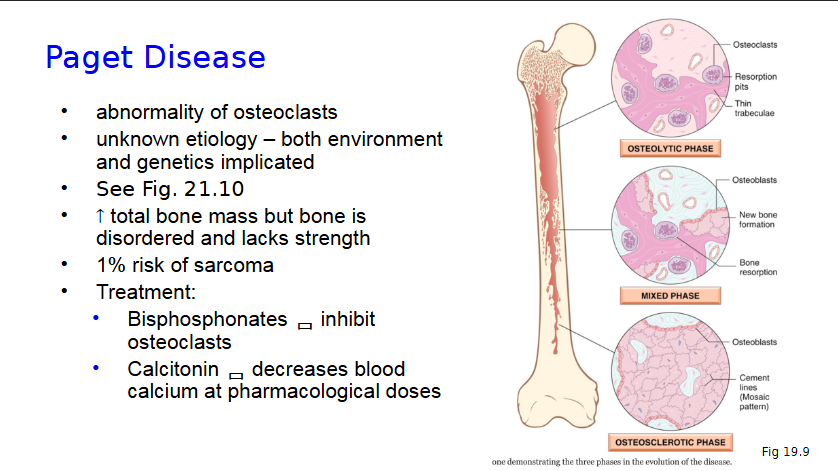
Paget Disease
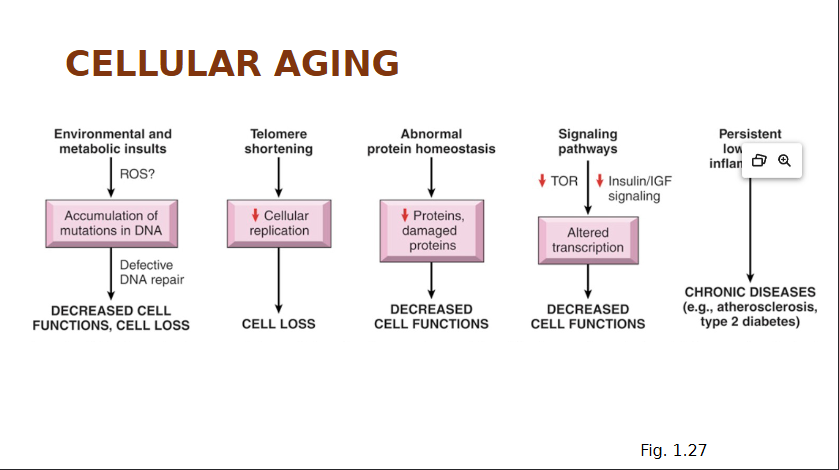
Cellular Aging
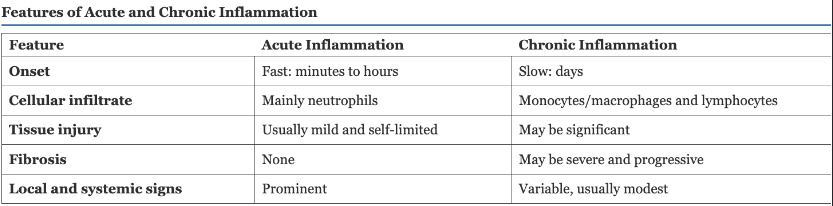
Acute Vs Chronic Inflammation
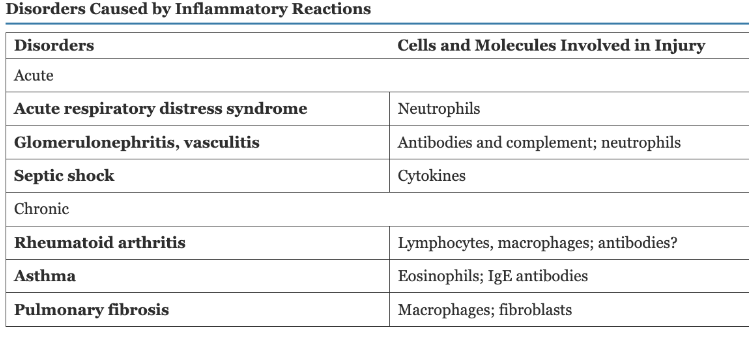
Disorders Causes by Inflammatory Reactions
What are the cardinal signs of inflammation?
Heat
Redness
Swelling
Pain
Loss of function
What are some of the causes of inflammation?
Infection
Cell Injury and tissue necrosis
Ischemia, physical and chemical injury
Foreign bodies
dirt, sutures, crystal deposits, splinters
Immune reactions
hypersensitivity reaction against
self tissues (AI diseases) or
Environmental substances (eg. Allergies)
How are microbes and damaged cells recognized?
Pattern Recognition Receptors
Recognize “danger signals” from many microbes or injured cells
2 Important Families
Toll-like Receptors (TLRs)
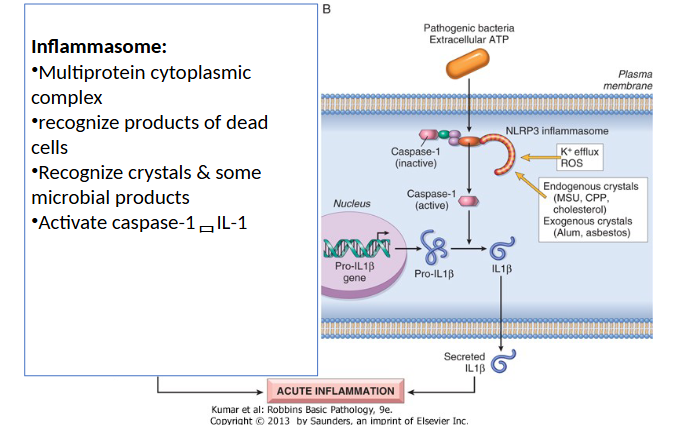
Inflammasome
TLR Recognition of microbes and damaged cells
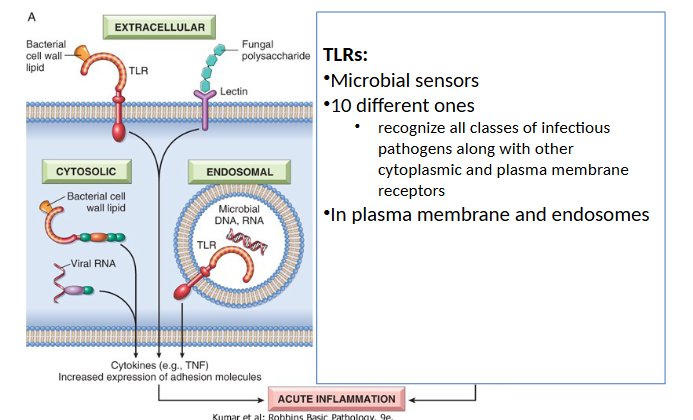
Inflammasome Recognition of microbes and damaged cells
are cytoplasmic and can recognize exogenous antigens and intracellular molecules
Main Features, Steps and Outcomes of Acute Inflammation
Main features:
vascular changes
cellular response
Steps:
Recognition of injurious agent
Recruitment of leukocytes
Removal of injurious agent
Regulation of response
Resolution (repair)
Outcome:
Elimination of injurious stimuli followed by tissue repair
Persistent Injury leads to chronic inflam
Vascular Changes include…
Vascular flow and diameter
Stasis
Vascular congestion & erythema
Leukocyte margination & recruitment
Increased vascular permeability
Lymphatic vessels & lymph node responses
What chemical mediators cause vasodilation in arterioles and what clinical signs show up as a consequence?
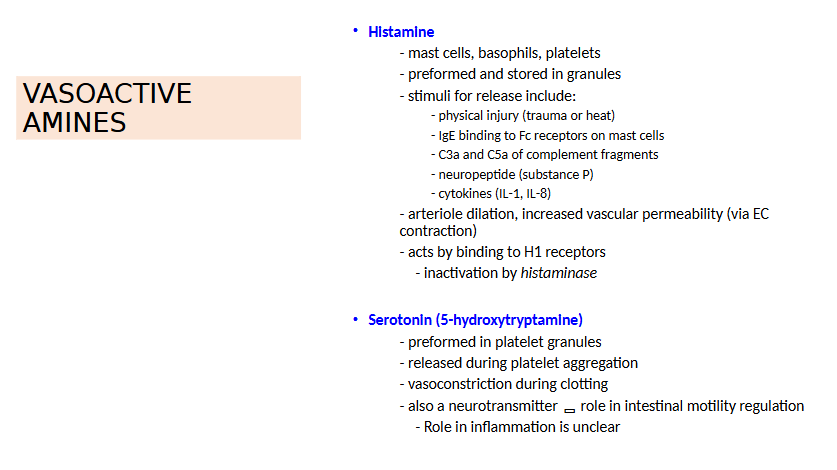
Histamine
NO
Increased blood flow causes Redness (erythema) & warmth
Vascular Permeability is caused by what THREE things
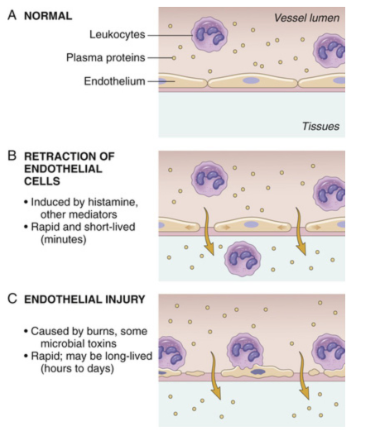
Retraction of Endothelial Cells
a. Increase Interendothelial spaces
b. Due to Inflam Mediators (eg. Histamine, bradykinin, leukotrienes) - rapid, transient
c. Cytokines (TNF, IL-1) - slower, prolonged response (Hrs)
Direct endothelial cell injury
a. neutrophils’ ROS Production
b. Burns
Increased transcytosis of proteins thru EC
a. Increased venular permeability
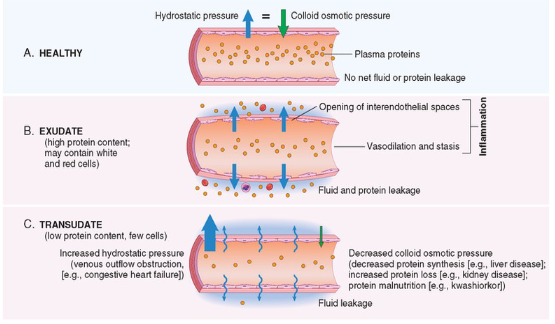
Increased Vascular Permeability causes what?
Edema, which is an increase in interstitial fluid
Protein-rich fluid (exudate) into extravascular tissues
Pus = purulent exudate
Inflammatory exudate with leukocytes, microbes and dead cells
Effects of Vascular Changes could include…
Changes in appearance of stasis (due to viscosity Increase and Decrease circulation)
Vascular congestion
Increased leukocyte accumulation along vascular endothelium margination
Lymphatic Vessel Responses In Acute Inflammation include…
Increased Transport
Lymphangitis
Secondary Inflam of lymph vessels
Characterized by red streaks in the skin
Lymphadenitis
Inflammation of draining lymph nodes
What are the two components of cellular response?
Leukocyte Recruitment
Leukocyte Activation
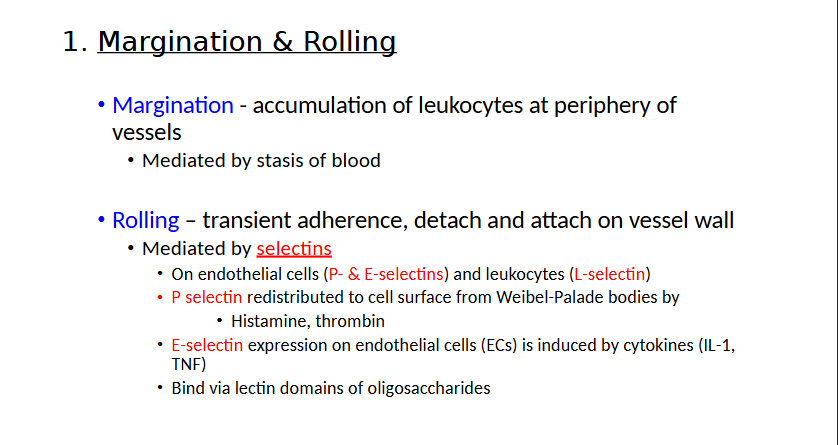
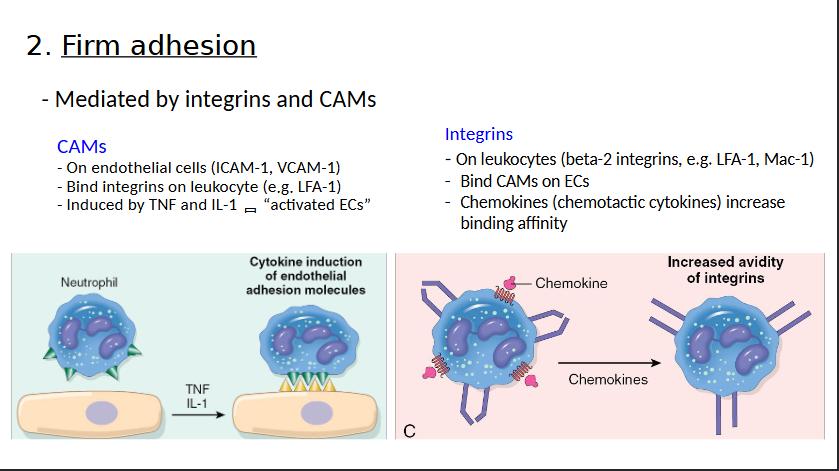
What are the four steps of Leukocyte Recruitment?
Margination & Rolling
Firm Adhesion
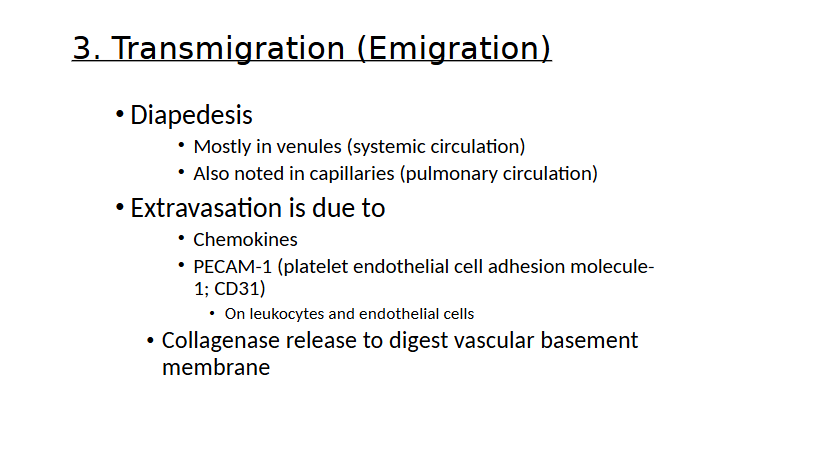
Transmigration
Chemotaxis
Margination and Rolling
Firm Adhesion
Transmigration (emigration)
Chemotaxis of Leukocytes

Leukocyte Activation
Recognition through receptors
Results in an enhanced
1. Phagocytosis
2. Killing and degradation of phagocytosed microbes
secretion of microbicidal agents from granules
ROS
Lysosomal enzymes
NETs
3. Inflammatory mediator release = amplify inflammatory response
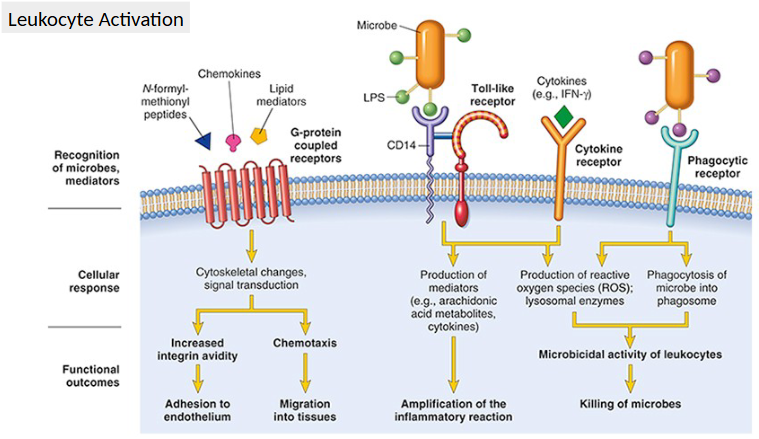
Intracellular Effects
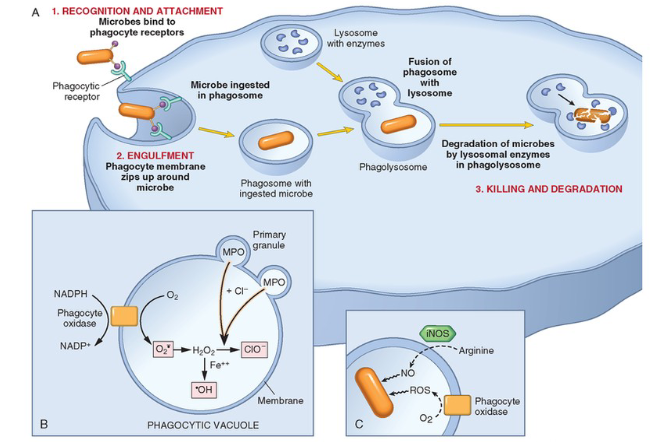
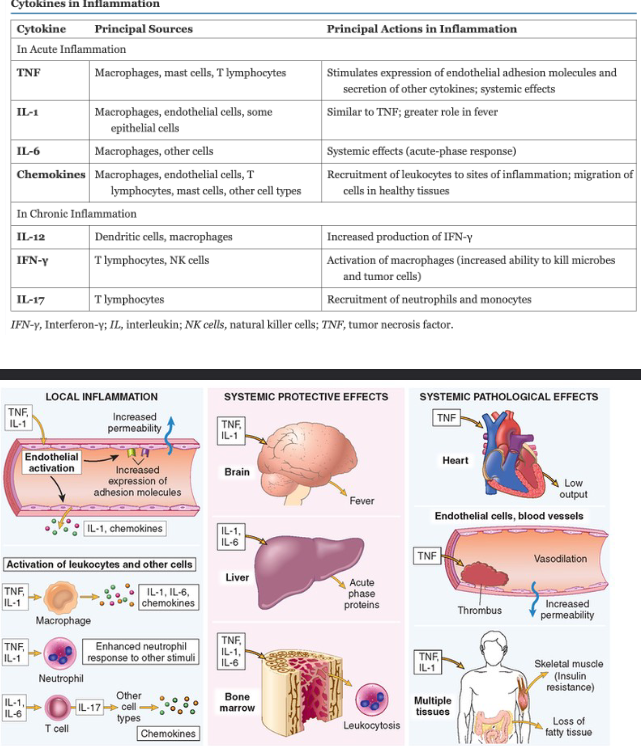
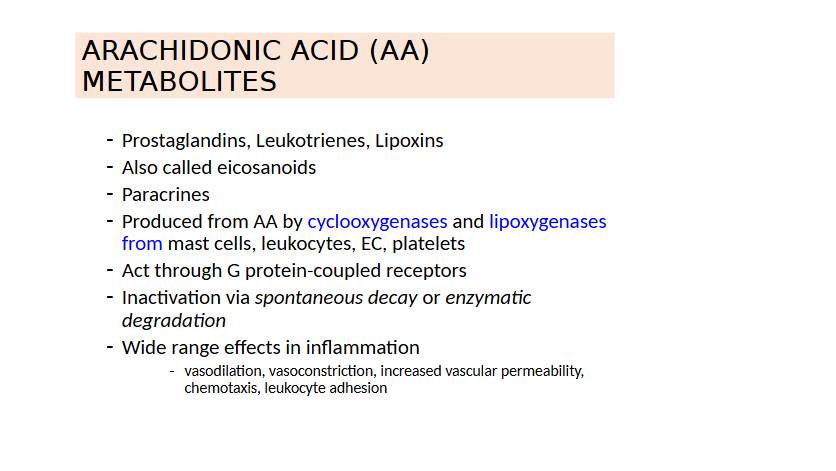
What are the General Properties and Chemical Classifications of Inflammatory Mediators?
The general properties are sourced as…
Cell-derived
Plasma-derived
Released/Activation Induced by Injury/Inflammation
Short Lived
Stimulate Other mediators
Chemical Classes
Vasoactive amines
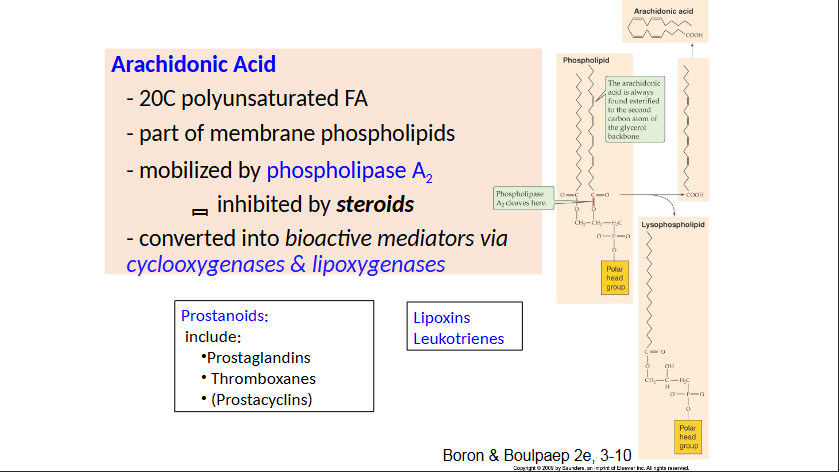
Lipid products (arachidonic acid metabolites)
Cytokines
Products of complement activation
Vasoactive amines
Arachidonic Acid Metabolites
Arachidonic Acid
Phospholipase A2 Pathways
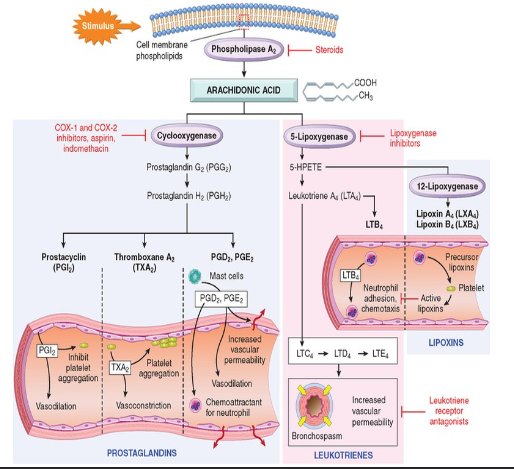
Principal Actions of Arachidonic Acid Metabolites in Inflammation
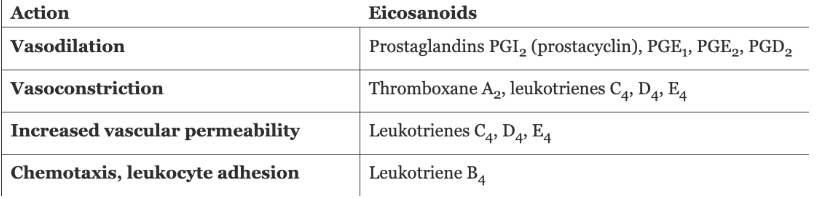
Prostanoid biosynthesis ***
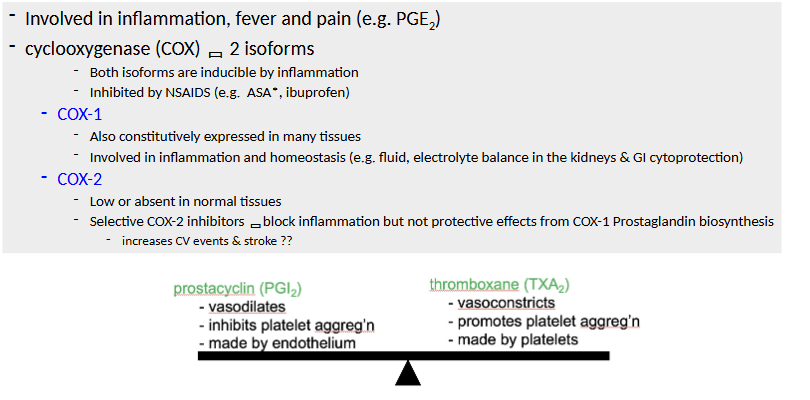
Leukotriene/Lipoxin Biosynthesis ***
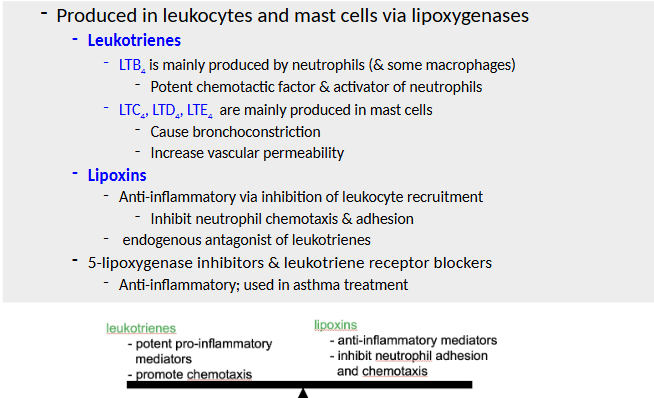
Corticosteroids
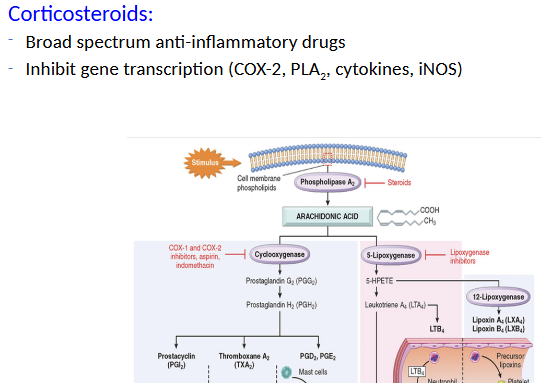
Cytokines and Chemokines
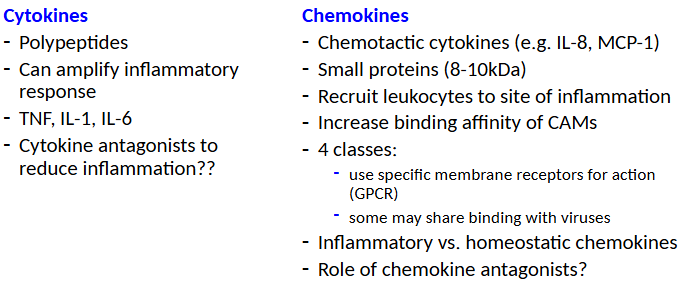
Cytokines and Inflammation Systemic Protective Effects and Pathological Effects