Acids and Bases
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Arrhenius Acid
Substance that produces H+ ions when dissolved in water
Arrhenius Base
Substance that produces OH- ions when dissolved in water
H+ ions are often
Protons
The chemical formula of the hydronium ion
H3O+
Bronsted-Lowry Acid
Substance that gives a proton to another substance (Proton Donor)
Bronsted-Lowry Base
Substance that accepts a proton from another substance (proton acceptor)
Lewis Acid
Electron pair acceptor
Lewis Base
Electron pair donor
Conjugate Acid
A base that gained a proton
Conjugate Base
An acid that has lost a proton
Conjugate Acid-Base pairs
Two substances related to each other by the transfer of a proton
The acid becomes its conjugate base by losing a proton
The base becomes its conjugate acid by accepting a proton
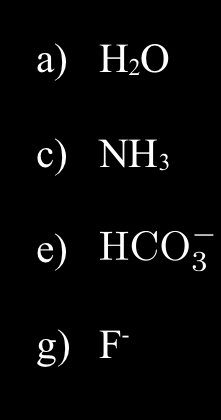
What is the formula for the Conjugate Acid?
a) H3O+
c)NH4+
e) H2CO3+
g) HF+
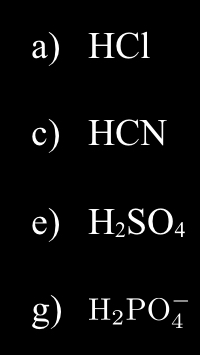
What is the formula for the Conjugate Base?
a) Cl-
c) CN-
e) HSO4
g)HPO4-
If the pH is under 7.0 is it Acidic, Basic or Neutral?
Acidic
If the pH is over 7.0 is it Acidic, Basic or Neutral?
Basic
If the pH is 7.0 is it Acidic, Basic or Neutral?
Neutral
Amphoteric
A chemical that can act as an acid or a base
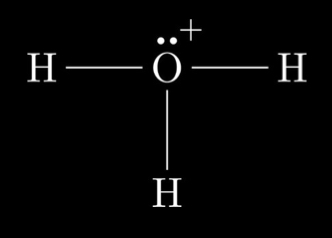
Lewis structure for the hydronium ion
H3O+
Strong Acid
Completely dissociate when dissolved in water
—> indicated complete dissociation in the chemical equation
Small reversible process but is ignored
List 7 Strong Acids
HI
HBR
HCI
HCLO3
HNO3
HCLO4
H2SO4
Weak Acid
Partially dissociate when dissolved in water
the dissociated form are in Dynamic equilibrium
Acid-Dissociation Constant (Ka)
Equilibrium constant for the dissociation of an acid
Strong acids have _______ Ka values and weak acids have _________ Ka values
LARGE, small
Strong acids have _______ pka values and weak acids have ______ pka values
small, LARGE
Both protons are considered strong acids in H2SO4? True or false
False
How many ionizable protons are in Monoprotic acids?
1 proton
How many ionizable protons are in Diprotic acid?
2 protons
How many ionizable protons are in Triprotic acids?
3 protons
The ionizable protons in polyprotic acids dissociate one at a time? True or False
True
Strong Base
Completely dissociate when dissolved in water
Name all 7 formula’s for Strong bases
LiOH
NaOH
KOH
Ca(OH)2
Sr(OH)2
Ba(OH)2
Strong bases are group _______ and group ________ hydroxides
1A and 2A
When multiple hydroxide ions are present, they dissociate one at a time? True or False
False
Weak Base
Produce OH- ions by accepting a proton from water
Many weak bases have a _______ atom with a _________________________
Nitrogen, lone pair of electrons that act like proton acceptor
What is the base dissociation constant?
Equilibrium constant for the ionization reaction of a weak base
If the element is a anion is it usually Basic, Acidic or Neutral
Basic
If the element is a cation is it usually Basic, Acidic or Neutral
Acidic
If an element is from Group 1A or 2A it always Basic, Acidic or Neutral
Neutral